Critical Reviews in Biotechnology, 22(3):245-279 (2002)
Botryococcus braunii: A renewable source of hydrocarbons and other chemicals
Anirban Banerjee,1 Rohit Sharma,1 Yusuf Chisti,2 and U. C. Banerjee1*
1Department of Biotechnology, National Institute of Pharmaceutical Education and Research, Sector 67, S.A.S. Nagar (Mohali) 160062, Punjab, India; 2Institute of Technology and Engineering, Massey University, Private Bag 11 222 Palmerston North, New Zealand* Corresponding author: Telephone: 91-172-214682, Fax: 91-172-214692, E-mail address: ucbanerjee@niper.ac.in
Table of contents
Abstract: Botryococcus braunii, a green colonial microalga, is an unusually rich renewable source of hydrocarbons and other chemicals. Hydrocarbons can constitute up to 75% of the dry mass of B. braunii. This review details the various facets of biotechnology of B. braunii, including its microbiology and physiology; production of hydrocarbons and other compounds by the alga; methods of culture; downstream recovery and processing of algal hydrocarbons; and cloning of the algal genes into other microorganisms. B. braunii converts simple inorganic compounds and sunlight to potential hydrocarbon fuels and feedstocks for the chemical industry. Microorganisms such as B. braunii can, in the long run, reduce our dependence on fossil fuels and because of this B. braunii continues to attract much attention.
Key words: Botryococcus braunii, microalgae, algal hydrocarbons, botryococcene, algal culture, photobioreactors.
© 2002 by CRC Press LLC
I. Introduction
The unicellular photosynthetic microalga Botryococcus braunii is member of the chlorophyceae (chlorophyta). Sequence analysis of the small subunit of r-RNA (16s RNA) and its comparison with the sequences for other algae suggests that B. braunii is closely related to Characium vaculatum and Dunaliella parva (Sawayama et al., 1995). This colonial microalga is widespread in fresh and brackish waters of all continents (Chisti, 1980). The cosmopolitan nature of the algae is confirmed by the strains originating in the USA (Wolf et al., 1985a, b), Portugal, Bolivia, France, Ivory Coast, Morocco, Philippines, Thailand, and the West Indies (Metzger et al., 1985a). These geographical regions belong to various climatic zones, including the continental, temperate, tropical, and alpine zones.
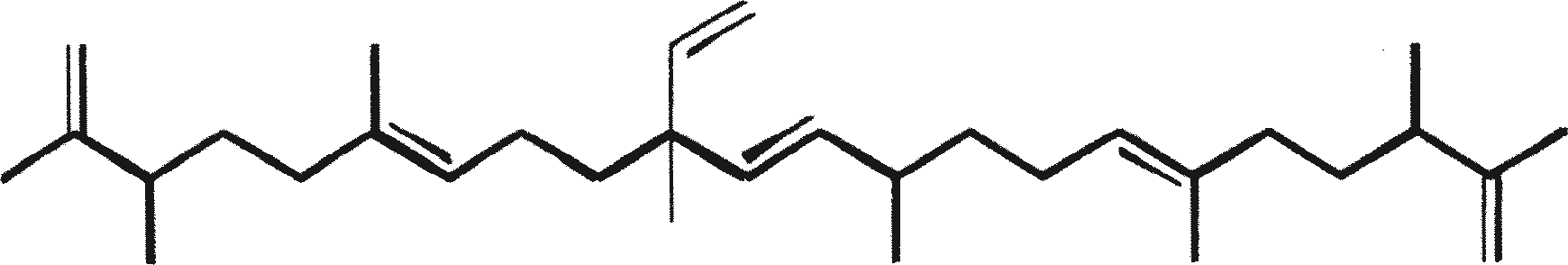
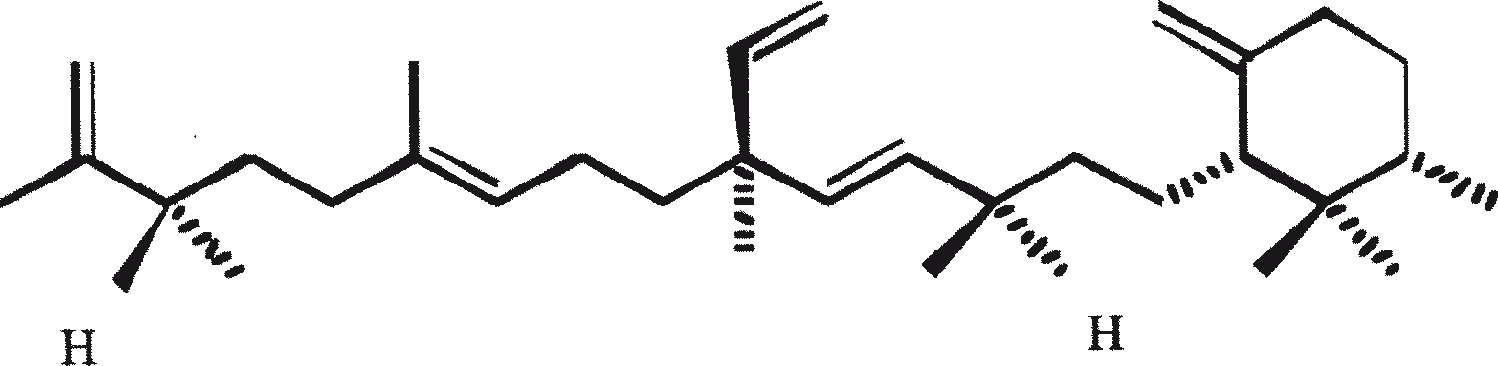
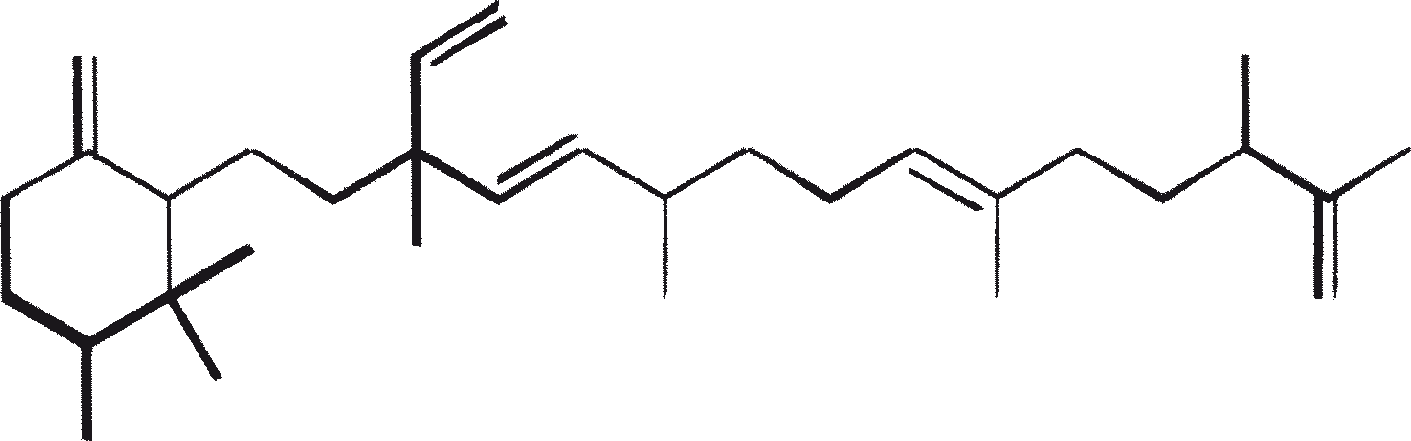
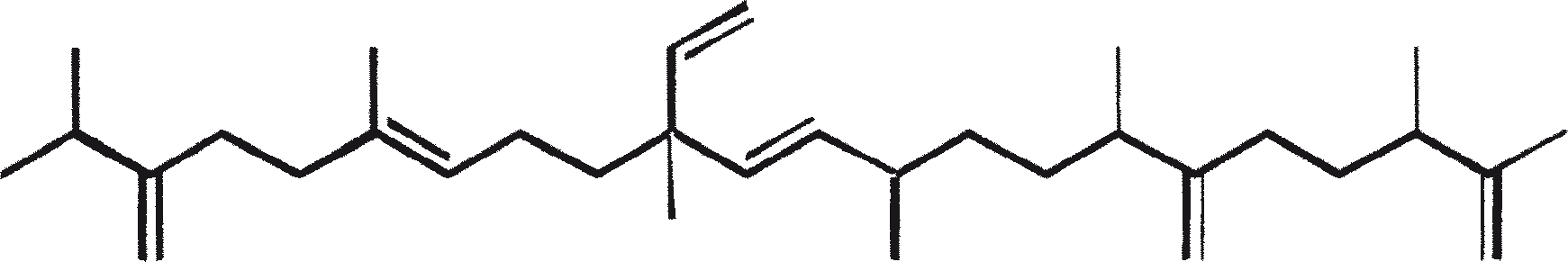
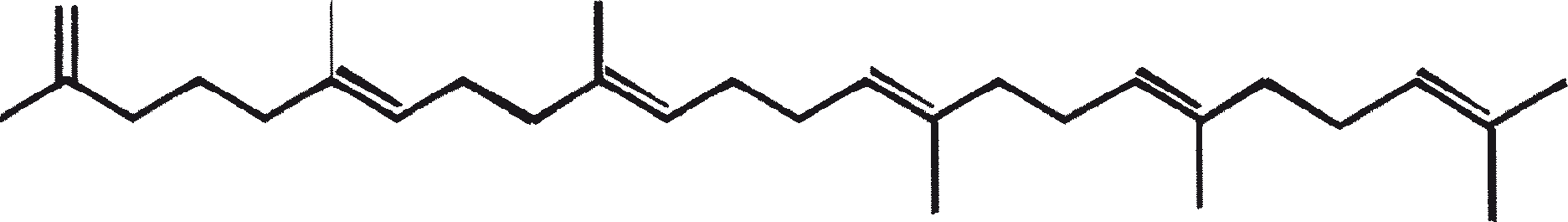
B. braunii is regarded as a potential source of renewable fuel because of its ability to produce large amounts of hydrocarbons. Depending on the strain and growth conditions, up to 75% of algal dry mass can be hydrocarbons. The chemical nature of hydrocarbons varies with the producer strain. Three races of B. braunii have been documented, and these can be differentiated on the basis of the characteristic hydrocarbon they produce. The A race produces oddnumbered C25 to C31, n-alkadienes, and trienes. The B race produces triterpenoid hydrocarbons known as botryococcenes (CnH2n-10, n = 30-37), apparently of isoprenoid origin (Chisti, 1980). The L race produces lycopadiene, a C40 tetraterpene.
Historically, interest in B. braunii arose because of its geochemical significance. Paleobotanical studies suggest that B. braunii is one of the major sources of hydrocarbon in a variety of oil-rich deposits dating from the Ordovician period to the present (Cane, 1977). Lycopadiene may be the precursor of lycopane found in some lacustrine sediment. The occurrence of botryococcane, C34H70, the hydrogenated derivative of botryococcene, at levels of 0.9 and 1.4% in two Sumatran crude oils has been reported (Moldwan and Seifert, 1980). These concentrations are the highest levels ever reported for a single complex fossil biological marker in petroleum (Moldwan and Seifert, 1980). Botryococcane has been found in a range of coastal bitumen (kerogen) formations in Australia (Mcirdy et al., 1986). B. braunii is the main source of C27, C29, C31 alkanes found in the Pula sediment, a picolene oil shale, of Hungary (Lichitfouse et al., 1994). Recently, a C30 botryococcene related compound, 7, 11-cyclobotryococca 5, 12, 26-triene was isolated in organic rich sediments from lake Cadagno, Switzerland, revealing the widespread nature of B. braunii in recent and past environments (Behrens et al., 2000).
Few oil shales contain botryococcene, even though B. braunii occurred widely during the formation of those shales. This apparent discrepancy is explained by degradation of botryococcenes in the oxic conditions of the early diagenesis (Derenne et al., 1988). Spectroscopic studies (FTIR, GC-MS, etc.) on unheated materials and pyrolyzates indicate that a predominant fraction of coorongite (75% of the bitumen free fraction), a rubbery deposit, is composed of the degradation-resistant biopolymers of the outer walls of B. braunii. These cell wall biopolymers are only marginally affected by the highly oxic conditions of coorongite formation.
B. braunii has been reported to convert 3% of the solar energy to hydrocarbons (Gudin et al., 1984). Being a photosynthetic organism, the alga can fix atmospheric CO2. Consequently, burning of algal hydrocarbons is not a net contributor to CO2 concentration in the atmosphere. The thermal value of biomass-derived hydrocarbons ranges between 30,000 and 42,000 kJ/kg (Held et al., 1985). Converting a 100-MW thermal power plant from using coal to using liquid fuel derived from B. braunii can reduce CO2 emissions by 1.5 × 105 tons/yr (Sawayama et al., 1999). Potentially, the CO2 emission values could be reduced further by improving the CO2 fixation efficiency of algae. A substantial effort is being devoted to achieving this goal.
This review discusses the microalga B. braunii; the synthesis of hydrocarbons and other biochemicals by the alga; the culture conditions for the production of hydrocarbons; the downstream recovery and processing of algal hydrocarbons; the cloning into other microorganisms of the B. braunii genes responsible for hydrocarbon synthesis.
II. Races of B. braunii
B. braunii exists in three different races: A, B, and L. The races can be differentiated generally on the basis of the characteristic hydrocarbons they produce. Race A produces C25 to C31, odd-numbered n-alkadienes and alkatrienes. These linear olefins can constitute up to 61% of the dry cell mass of the green active-state colonies (Gelpi et al., 1970). Two unusual hydrocarbons, C27H51 triene and C27H48 tetraene, were isolated from a B. braunii strain originating in Lake Overjuyo, Bolivia.
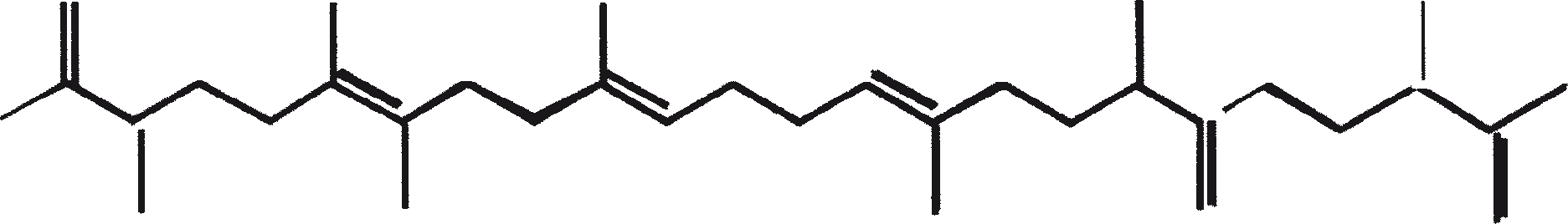
The B race produces polymethylated unsaturated triterpenes, called botryococcenes (CnH2n-10, n = 30-37). Botryococcenes can exist as isomers with the same number of carbons but different structures. In natural populations, botryococcene can constitute from 27 to 86% of the dry cell mass (Brown and Knights, 1969). The L race produces a single hydrocarbon C40H78, a tetraterpene, known as lycopadiene (Metzger et al., 1990). This hydrocarbon accounts for 2 to 8% of the dry biomass (Metzger and Casadevall, 1987).
The three races can be differentiated on the basis of certain morphological and physiological characteristics (Table 1). The cells of the L race (8 to 9 µm × 5 µm in size) are relatively smaller than the cells of races A and B (13 µm × 7-9 µm) (Metzger et al., 1988). The L race also contains less pyrenoid (Metzger et al., 1988). Another difference among the races is the colony color in the stationary phase. The B and L race algae turn red-orange and orangebrownish from green color of the active state. The A race alga turns pale yellow from green. This difference is due to the accumulation of keto-carotenoids (canthaxanthin, echinenone, adonixanthin, etc.) in the stationary phases of the B and L races.
Distinctive features of races of B. braunii
| B. braunii | |||
| Race-A | Race-B | Race-L | |
| Nature of hydrocarbon | C25-C31 odd numbered n-alkadienes/trienes | Botryococcenes (triterpenes) CnH2n-10, n = 30-37 |
Lycopadienes (tetra-Terpene) C40H78 |
| Colony color in stationary phase | Pale yellow or green | Orange-reddish or orange-brownish due to accumulation of carotenoids | |
| Long chain alkenyl phenols | Present | Absent | Absent |
| Nature of biopolymers | Very long aliphatic chains cross-linked by ether bridges and bearing fatty esters | Tetraterpenoid cross-linked by ether bridges | |
The races can also be distinguished on the basis of the nature of the biopolymers present in the cell wall. The A and B races contain long aliphatic compounds cross-linked by ether bridges. In contrast, the basic structure of the biopolymer of L race is based on lycopadiene cross-linked by ether bridges. Long chain alkenyl phenol is detected in the A race. The role of this aliphatic chain is to increase the solubility of phenolic moiety in the lipidic region, a requirement for protection against biological and chemical oxidative degradation. The lipids of B and L races consist of terpenoids that are more resistant to bacterial attack than are the aliphatic hydrocarbons (Metzger and Casadevall, 1989). Although biochemically different, the various races coexist in natural environment. Mixed populations of alkadiene and botryococcene races have been found in Australian lakes (Wake and Hillen, 1981). The characteristic hydrocarbons produced by races A, B, and L of B. braunii are noted in Tables 2a, 2b, and 2c, respectively. Because of a generally higher hydrocarbon content, races A and B may be superior to race L for commercial extraction of hydrocarbon fuel.
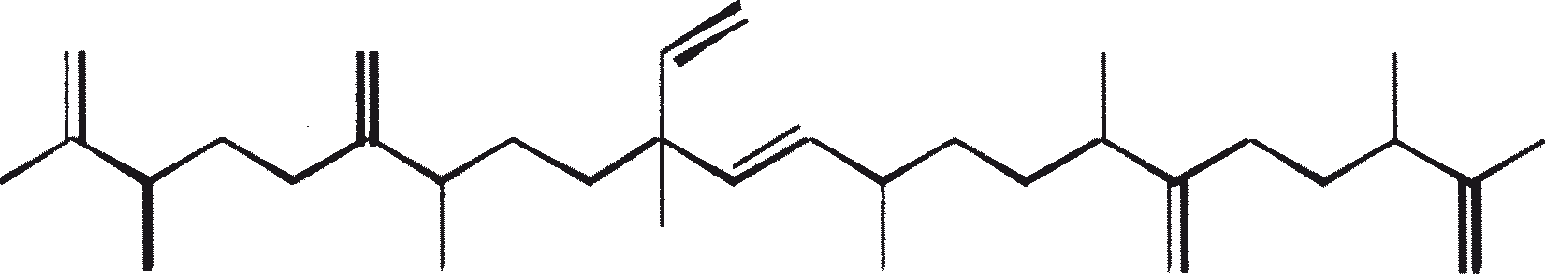
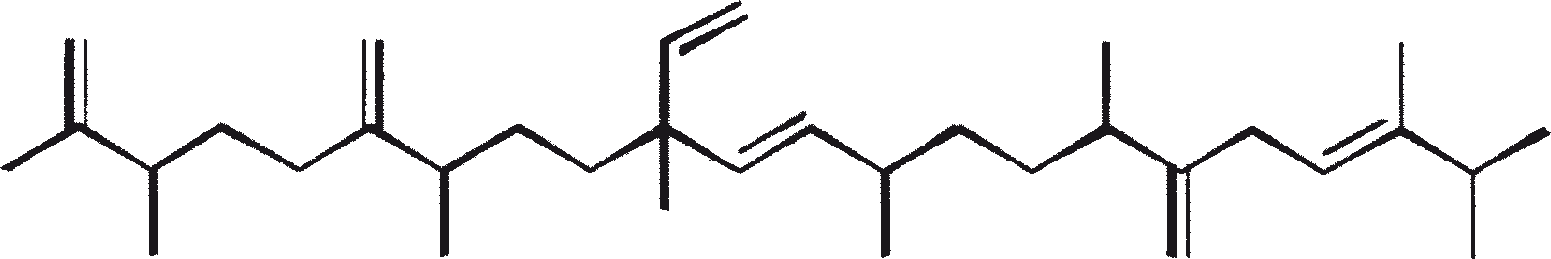
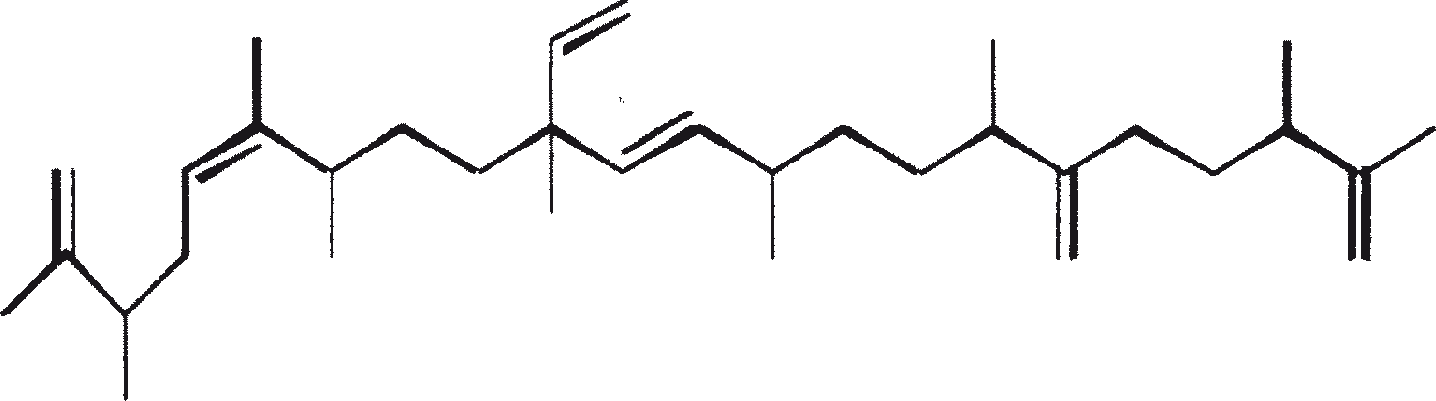
The major characteristic hydrocarbons produced by race A of B. braunii
| Hydrocarbons | Structure | Position of double bond | |
| 1 | C25H48 (Pentacosa-1, 16-diene) |
CH3(CH2)7CH=CH(CH2)13CH=CH2 | 1, 16(Z). 1, 16(E) |
| 2 | C27H52 (Heptacosa-1, 18-diene) |
CH3(CH2)7CH=CH(CH2)15CH=CH2 | 1, 18(Z) 1, 18(E) |
| 3 | C29H56 (Nonacosa-1, 20-diene) |
CH3(CH2)7CH=CH(CH2)17CH=CH2 | 1, 20(Z) 1, 20(E) |
| 4 | C31H60 (Hentriaconta-1, 22-diene) |
CH3(CH2)7CH=CH(CH2)19CH=CH2 | 1, 22(Z) |
| 5 | C29H54 (Nonacosa-1, 20, 22-triene) |
CH3(CH2)5CH=CH-CH=CH-(CH2)17CH=CH2 | 1, 20(Z), 22(Z) |
| 6 | C31H58 |
Not yet elucidated | |
| 7 | C27H51 |
E/Z E/Z CH3(CH2)7CH=CH(CH2)13CH=CH-CH=CH2 |
|
| 8 | C27H48 |
E/Z E E/Z CH3(CH2)7CH=CH(CH2)11CH=CH-CH=CH-CH=CH2 |
|
| (No. 7 and 8 are the unusual hydrocarbons produced by race A of B. braunii isolated from Overjuyo lake, Bolivia) | |||
III. Biosynthesis and accumulation of unsaturated hydrocarbons
B. braunii A race produces nonisoprenoid hydrocarbons alkadienes and trienes. The structural similarity (locations of double bonds), and stereochemistry of alkadienes resemble that of oleic acid (18:1, cis-ω9). These features and other evidence suggest that oleic acid is the direct precursor of the n-alkadienes. The intracellular concentration of oleic acid remains relatively low during rapid production of hydrocarbons (e.g., in exponential and early linear phases of growth), but when hydrocarbon production declines (deceleration phase) the oleic acid concentration rises sharply (Templier et al., 1984). Other factors (e.g., occurrence of a terminal double bond in the hydrocarbons; inhibition of production by the presence of dithioerythritol, DET) suggest that the synthesis of hydrocarbons (dienes) proceeds via an elongation-decarboxylation route rather than a head-to-head condensation pathway. In the elongation-decarboxylation mechanism, oleic acid acting as a direct precursor is elongated by successive addition of C2 units derived from malonyl Co-A until the appropriate chain lengths are attained. These very long chain derivatives are then decarboxylated to give hydrocarbons that are released from elongation-decarboxylation complex (Templier et al., 1984).
The elongation-decarboxylation system of B. braunii is nonspecific and can accept both oleic acid and elaidic acid (18:1, trans-ω9) to produce both cis and trans dienes. An isomerization system exists that converts oleic acid to elaidic acid. This isomerization step is slow relative to transformation of elaidic acid to trans alkadienes, and this prevents accumulation of elaidic acid in the algal strains that produce trans-dienes. The strains lacking in trans dienes (Aus strain) are unable to produce elaidic acid from oleic acid via isomerization (Templier et al., 1991a, b). Synthesis of very long chain monoenic cis, trans-ω9 fatty acids also occurs via a non-specific elongation-decarboxylation system. This system needs a large endogenous pool of the corresponding acids, and it is completely different from the nonspecific elongation-decarboxylation system that produces hydrocarbons (Templier et al., 1984). Decarboxylation is the last step of hydrocarbon biosynthesis. Decarboxylation has a high activation energy and needs the presence of some activating group to facilitate the elimination of carbon dioxide. The required activation energy may be provided by a β-OH group present in the long chain fatty acyl derivatives (Yong et al., 1986).
The alkatrienes with one terminal and two cis-ω7, cis-ω9 unsaturations are synthesized by an elongation-decarboxylation mechanism, which is selective. This selective mechanism does not accept linoleic acid (cis-ω6, cis-ω9, 18:2) as a precursor. Growth in the presence of large amounts of exogenous linoleic acid does not induce the production of corresponding trienes. Trienes are also unlikely to be formed via desaturation of corresponding dienes, as confirmed by feeding the cells with radiolabelled dienes. Oleic acid is also the direct precursor of trienes. The additional double bond is introduced probably at the early stages of elongation-decarboxylation process, either via direct desaturation of oleic acid or desaturation of very long chain fatty acyl derivatives produced by elongation of oleic acid (Templier et al., 1991a).
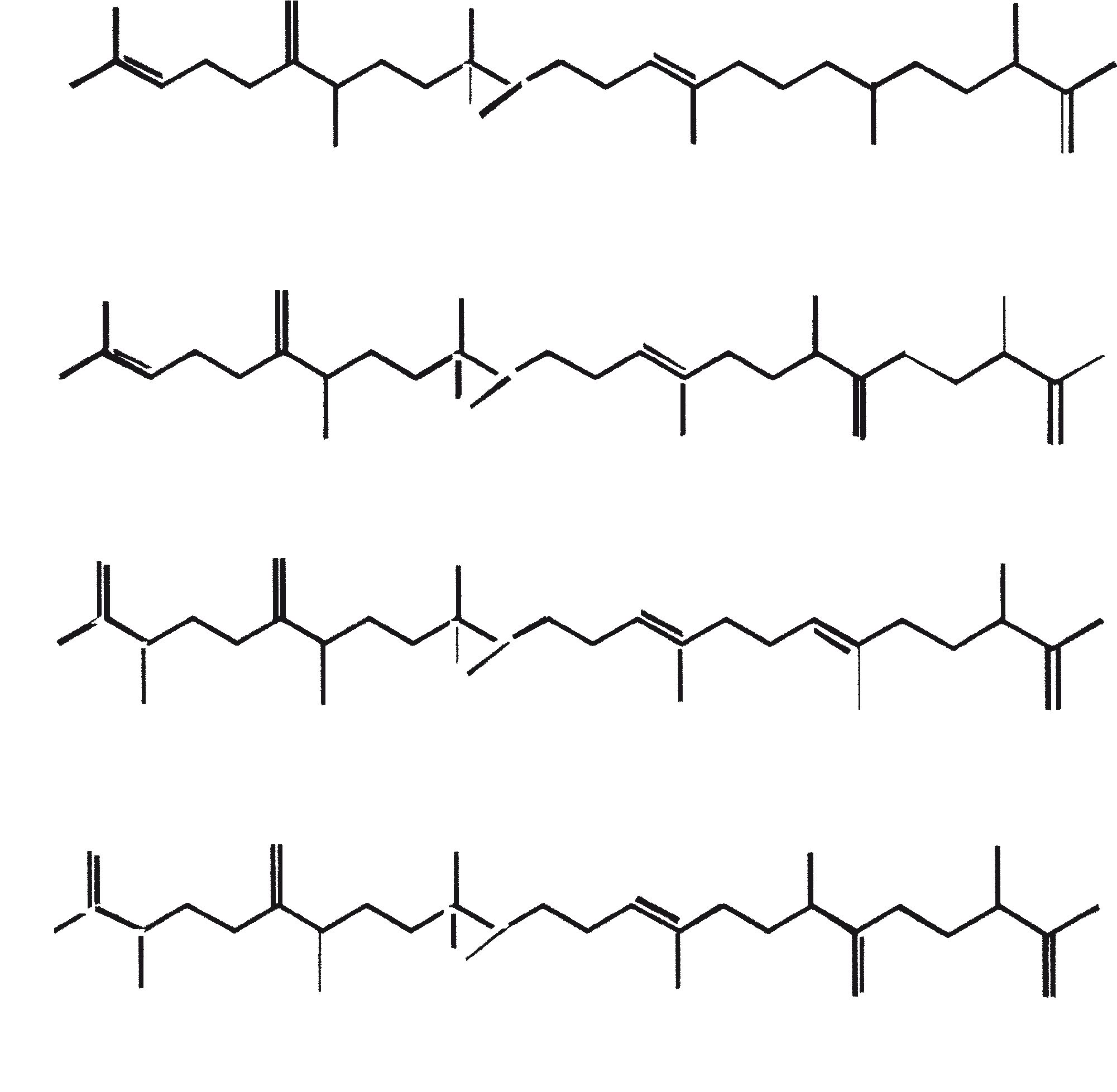
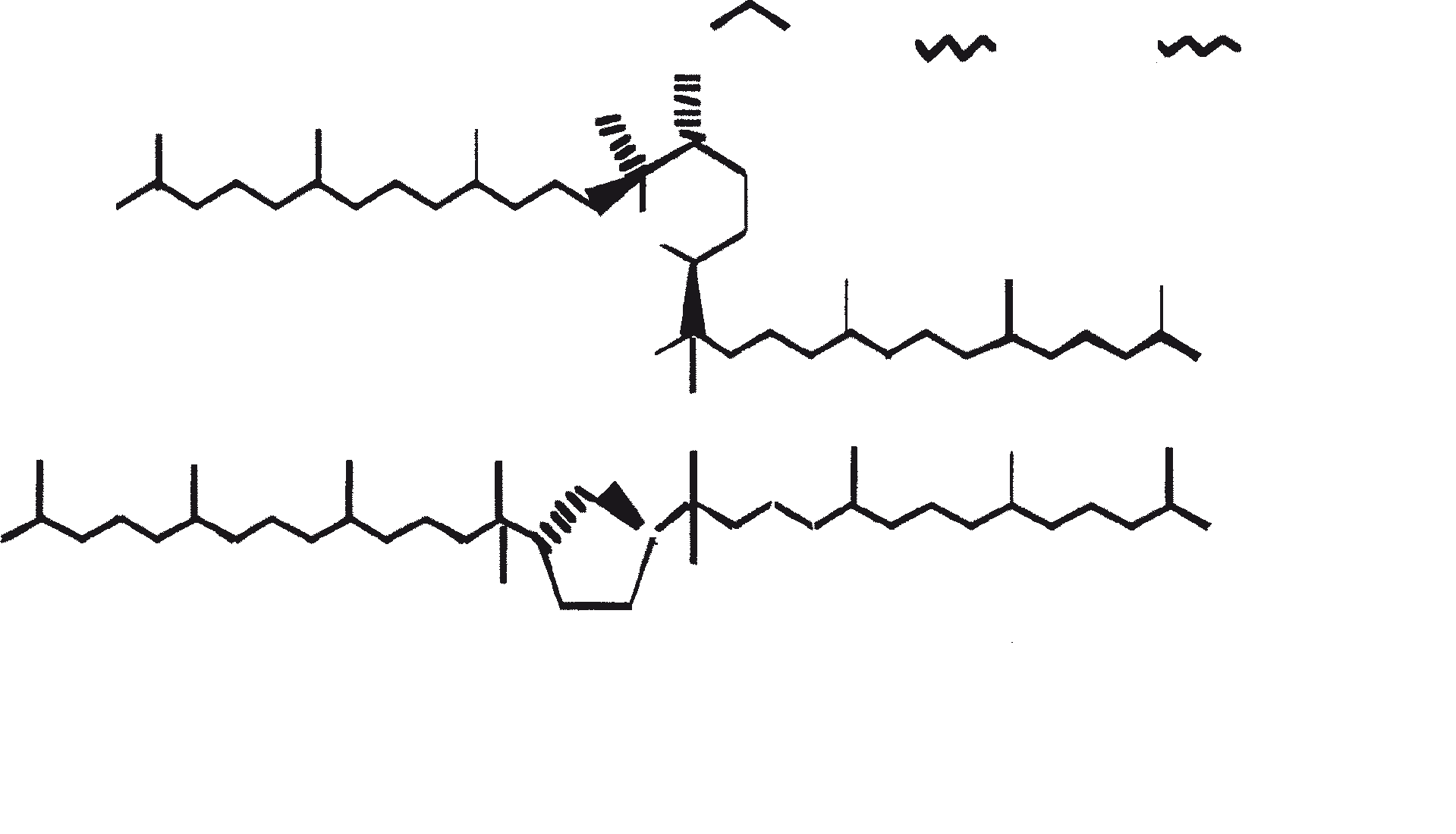
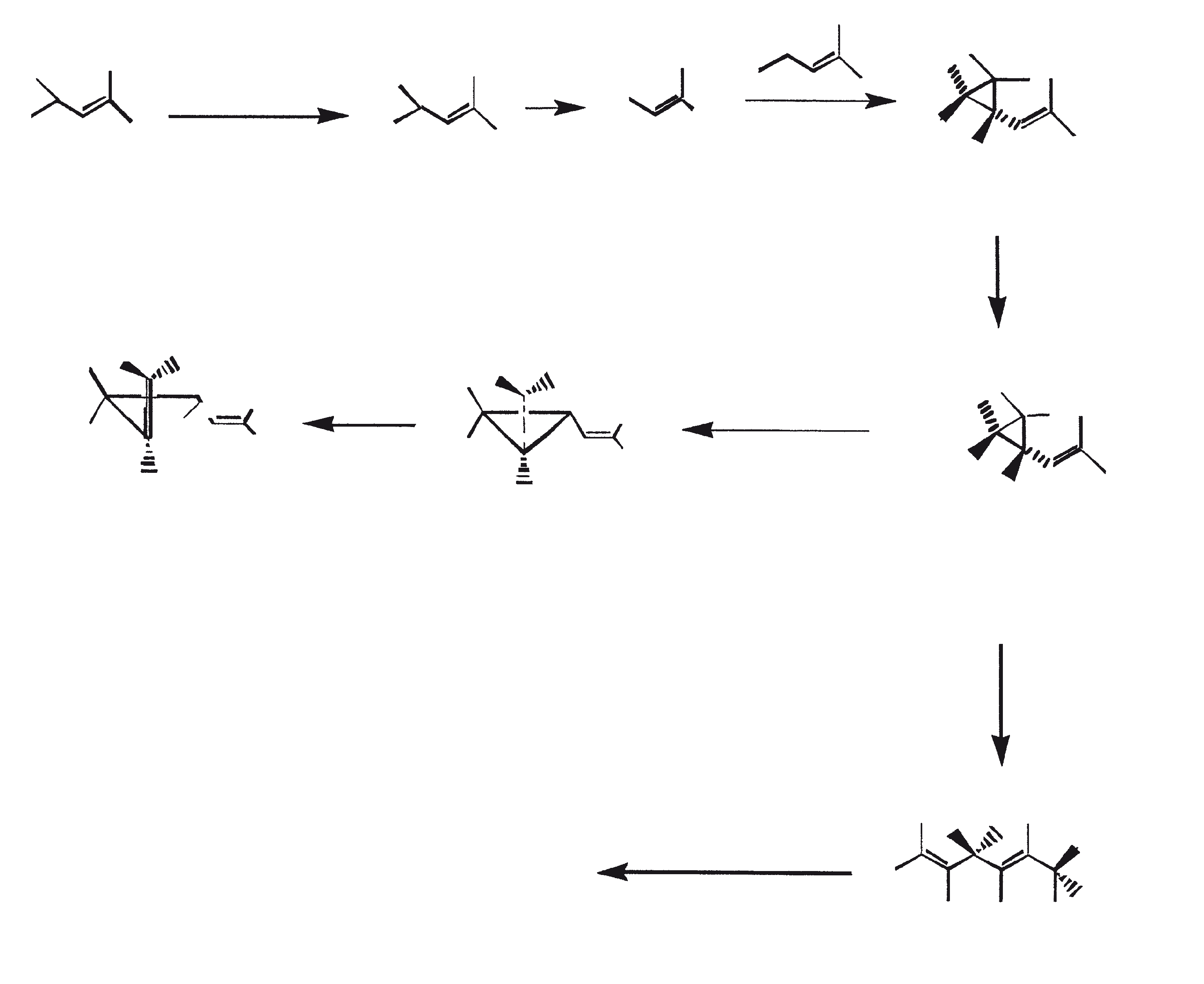
Race B produces isoprenoid compounds with an unusual 1'-3 fusion of isoprene units in the center of the chain. These compounds are known as botryococcenes. Several biosynthetic mechanisms for botryococcenes have been proposed. Studies suggest that the C30 botryococcene, the parent molecule for the higher homologues, is constructed from two molecules of farnesyl diphosphate, generated from farnesol by the action of farnesol phosphokinase (Inone et al., 1994a, b). Presqualene diphosphate, derived from the farnesyl diphosphate via carbene addition to the olefenic double bond (Griessman, 1969), is a member of chrysanthemyl species (1'-2-3 fused compounds). The cyclopropylcarbinyl cation generated by the release of pyrophosphate leads to 1'-3 (artemisyl), 2-1'-3 (santolinyl), and 1'-1 (head-to-head) structures. In vitro solvolysis studies suggest that 98% of the products have a artemisyl carbon skeleton (Poulter et al., 1977). When a single reactive intermediate can lead to more than one product, the predominance of a specific isomer suggests the presence of an enzyme for regulating the regiochemistry of the intermediate.
Botryococcenes belonging to the artemisyl structure family are formed by a rearrangement of cyclopropylcarbinyl precursor, in this case the C30 cation (R=C11H19), in a reaction terminated by transfer of hydride from NADH or NADPH to the allylic cation (Poulter et al., 1977). Squalene is also derived from the same precursor via rearrangement and hydride capture but 1'-1 fusion. Botryococcenes are favored over squalenes because of the more facile rupture of 1'-2 bond. A comparison of biosynthesis of botryococcenes and squalene indicates that the relative positions of the C30 substrate and nicotinamide co-factor are similar in the enzyme substrate complex of squalene synthase and botryococcene synthase (Huang et al., 1988). A logical inference from this is that the enzymes catalyzing these reactions may share some common structural motifs, including those important for substrate binding and possibly catalysis.
Mutational analysis of amino acids in domains III, IV, and V of the squalene synthase suggests that these regions are essential for the enzyme activity. Asp219 and Asp223 of domain IV appear to be responsible for binding of the diphosphate moiety of farnesyl diphosphate (FPP) through magnesium salt bridges. Tyr171 in domain III is essential for the first step of the reaction. An accumulation of presqualene diphosphate (PSPP) in mutants of Phe288 of domain V indicates that this domain is involved in the second step of the reaction and possibly in the cleavage of the cyclopropane ring to form 1'-1 linkage.
On the other hand, a synthesis of botryococcenes requires facile rupture of the C(1') -C(2') cyclopropane bond (Gu et al., 1998). A possible explanation for the apparent discrepancy between biomass accumulation and squalene synthase activity is that the culture cycle consists of two growth phases. The first phase would be associated with cell proliferation and accumulation of squalene for sterol and membrane biogenesis and a limited amount of squalene for extracellular biopolymer assembly. The early induction of squalene synthase could be sufficient to account for that need. The second phase of biomass accumulation is correlated with maturation of cells and a dedication of their biosynthetic capacities to the accumulation of botryococcenes.
Given the common mechanistic features, it is not unreasonable to think that botryococcenes and squalene might be derived from a squalene synthase-like reaction (Jarstfer et al., 1996; Zhang and Poulter, 1995) catalyzed by a single enzyme that subtly modifies to yield either one of the two products; however, two different enzymes may be involved (Figure 1). Also, the alternative procedures involving farnesal and 3-hydroxy-2, 3-dihydrofarnesal (both derived from farnesol by the action of farnesol hydratase) acting as precursors (aldol condensation followed by rearrangement or Michael Addition) cannot be overruled (Inone et al., 1993).
synthase
(?)
and
NADPH*
Figure 1. Mechanism of biosynthesis of botryococcenes and squalene by B. braunii.
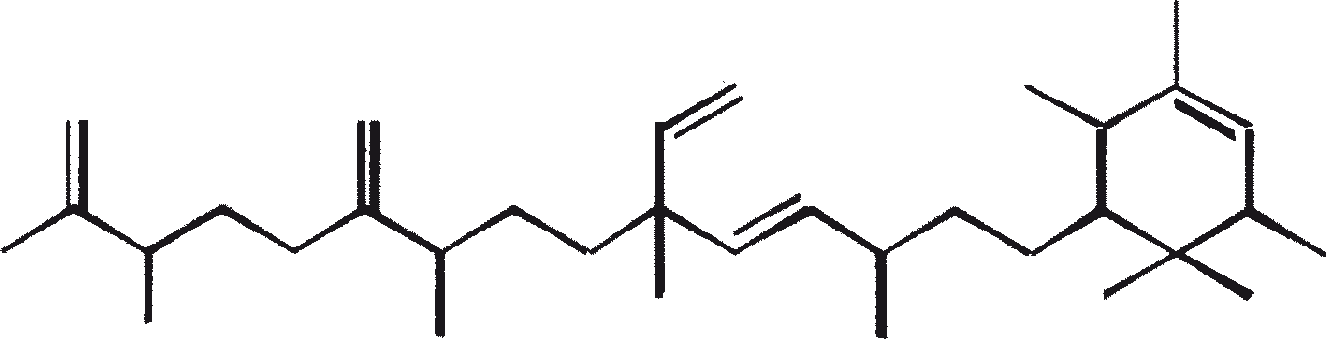
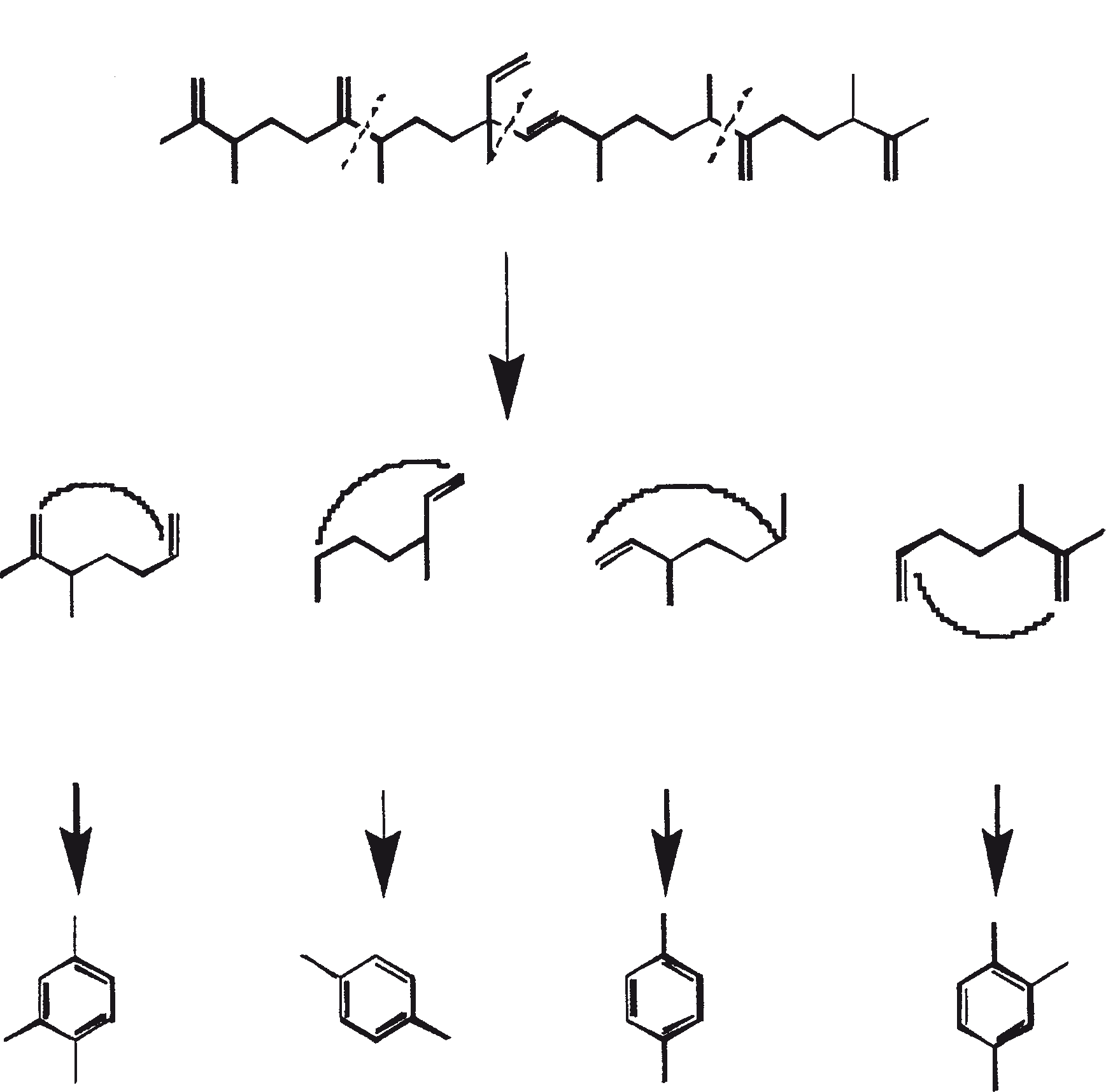
The C30 botryococcene acts as the precursor for higher botryococcenes. Mono methylations occur in most cases on the carbons 3, 7, 16, and 20, other positions involved are 18 (for 12), 22 (for 15), and 30 (for 16) (Metzger et al., 1985b). S-adenosyl methionine is reported to be the methylating agent (Wolf et al., 1985b). Pulse chase experiments suggest that methylations occur one at a time in a sequential manner, and the reaction occurs both in the cell and in the extracellular matrix (Metzger et al., 1987). In view of the richness of methylation patterns observed among this family of hydrocarbons, it is quite possible that methylases in botryococcene pathway have low substrate specificities. The alga does not seem to have a large family of highly specific methylases. This is supported by the fact that when the cells are cultivated in presence of high levels of methionine, large amounts of methylated squalenes are produced. This occurs because nonspecific methylases convert squalene to methylated squalenes, which divert from the normal sterol biosynthesis and accumulate with other hydrocarbons (Huang and Poulter, 1989). Cyclic isomers occur probably because of alkylation and the resulting cyclohexyl cation rearranging to olefenic structure (Figure 2) (David et al., 1988; Metzger et al., 1985b; Murakami et al., 1988).
Biosynthesis of lycopadienes is assumed to proceed via tail-to-tail combination of two phytyl units; however, a lycopersene reduction mechanism cannot be excluded (Metzger and Casadevall, 1987). Raman spectroscopy and electron microscopy show that hydrocarbons accumulate internally in cytoplasmic inclusions (4%) and externally in successive outer walls (95%) (Largeau et al., 1980). Approximately 2% of the hydrocarbons occur as free globules in the culture medium (Largeau et al., 1980). Hydrocarbons in the external pool can be recovered by short-contact extraction with solvents such as hexane. The hydrocarbons of the external and internal pools are structurally the same, but their relative abundance differs in these pools. Long chain hydrocarbons predominate in the external pool.
For the B race, an excretory process exists for botryococcenes from the cell (Metzger et al., 1987). Higher botryococcenes (C34, C36, etc.) accumulate in the external pool, whereas the internal pool consists of the lower homologues (C30, C31, etc.). In addition to the aforementioned external and internal pools where the hydrocarbons are stored irreversibly, a separate metabolic pool exists. In the A race, generation of alkenyl epoxides from the alkadienes of the metabolic pool is a normal occurrence. However, when the cells are incubated with exogenous alkadienes, increasing amounts of alkenyl epoxide and alkadienones are formed because of an abnormally large increase in the size of the metabolic pool caused by diffusion of exogenous alkadienes (Templier et al., 1992a).
IV. Cell physiology and the extracellular matrix
The cells of B. braunii are embedded in a communal extracellular matrix (or “cup”), which is impregnated with oils and cellular exudates (Blackburn, 1936). Cells are attached to each other by a refringent material that sometimes links two or more distinct clumps of cell. The wall of each cell possesses an internal fibrillar layer made of polysaccharide and an external trilaminar sheath (TLS) (Largeau et al., 1980). After cell division, each cell secrets new cups within the cup of the mother cell. Thus, the rubbery matrix appears to be composed of successive cups saturated with oil. Cells at the end of the exponential phase contain numerous chloroplasts with thylakoids arranged parallel to the cell surface and very few starch granules present. The cytoplasm is filled with lipid globules (Metzger et al., 1985a, b).
During cultivation, B. braunii undergoes a color change because of an accumulation of secondary carotenoids (carotenoids produced in large quantity under stress conditions such as nitrogen deficiency and high light intensity) in the matrix. The presence of carotenoids is more pronounced in races B and L. In the linear stage of growth, both these races produce almost equal amounts of β, β-carotene, echinenone, 3-OH echinenone, canthaxanthin, lutein, violaxanthin, loroxanthin, and neoxanthin. Whereas lutein is the major carotenoid in the linear phase (22% and 29% of total in B and L races, respectively), canthaxanthin (46%) together with echinenone (20% and 28%) are the dominating carotenoids in the stationary phase (Grung et al., 1989). Adonixanthin is also present in substantial amount in the L race in stationary phase (Grung et al., 1994). The main component of extracellular carotenoids is a ketocarotenoid, echinenone. The color change is mainly due to accumulation of echinenone in the intracellular matrix and a simultaneous decrease in the amount of intracellular pigments (Tonegawa et al., 1998).
Carotenoid biosynthesis follows the same pathway as botryococcenes’ but with a higher carbon number precursor, geranyl-geranyl pyrophosphate (Poulter and Hughes, 1977). Some newly identified carotenoids, for example, botryoxanthin-A (Okada et al., 1996), botryoxanthin-B, and α-botryoxanthin-A (Okada et al., 1998), braunixanthin 1 and 2 (Okada et al., 1997)—isolated from the B race may contribute to the color of the algal colonies. These new carotenoids are structurally related to squalenes.
The outer wall of B. braunii is composed of a biopolymer (9 to 12% of dry cell mass) that is highly resistant to nonoxidative chemical degradation, especially acetolysis. The biopolymer sometimes resembles sporopollenins because of their modes of deposition, resistant nature, and UV fluorescence. Sporopollenins are a class of biopolymers originating from oxidative polymerization of carotenoids and/or carotenoid esters (Goodway et al., 1973). Sporopollenins make outer walls of spores and pollens and also occur in some algal, bacterial, and fungal cell walls. However, IR and 13C NMR studies reveal that the resistant polymer of B. braunii is not derived from carotenoids and hence cannot be considered to be sporopollenin. All the three races of B. braunii contain resistant polymer and this polymer, may be the reason for coorongite formation. A similarity exists between races A and B in the nature of constituents of the biopolymer.
Long hydrocarbon chains (up to C31), saturated and unbranched, probably linked via ether bridges, make up the polymeric networks of resistant polymers PRB-A (A race) and PRB-B (B race). Hydroxyl groups and ester groups also occur in the biopolymer, but they are effectively protected by the three-dimensional network of hydrocarbon chains. Consequently, the esters are not affected by drastic saponification and acidic treatments. Both PRB-A and PRB-B exhibit high hydrogen and low nitrogen, phosphorus, and oxygen content. Comparative analysis shows both PRB-A and PRBB are composed of C24 to C30 very long chain fatty acid derivatives (VLCFA) originating from oleic acid. Feeding experiments and inhibition with dithioerythritol (DTE) suggest that characteristic hydrocarbons produced by race A and PRB-A share some common biosynthetic pathway. On the other hand, no biosynthetic relationship exists between PRB-B and botryococcenes, in the B race. Thus, lower labeling of PRB-A occurs when B. braunii (race A) is grown in externally added oleic acid (labeled), reflecting diversion of oleic acid toward hydrocarbon production (Lareillard et al., 1988).
The presence of SC1058 (a cinnolinyl acid derivative [1-N-benzyl-3-carboxy-4-keto cinnoline]) in growth medium inhibits the synthesis of biopolymer in both A and B races. SC1058 affects the ratio of the external and internal lipid pools. This effect is much more pronounced in case of A race than B race, reflecting a strong relationship between the hydrocarbons in A race and PRB-A biosynthesis (Templier et al., 1993). It is suggested that SC1058 inhibits the transformation of VLCFA derivatives to hydrocarbons by decarboxylation. SC1058 also affects the transport of lipids from internal to external pool (Templier et al., 1992b). The major constituents of the resistant biopolymer (PRB-A and PRB-B) are botryals, alkenyl epoxides, alkenyl phenols, epoxy botryals, alkenyl resorcinols, etc., and the condensation products between them and alkadienes, alkatrienes, or long chain fatty acids. The mechanism of biosynthesis of PRB-A and PRB-B by B. braunii is given in Figure 3. The cell wall-associated hydrocarbons produced by the various races of the alga are shown in Table 3.
derivatives
(w9, w23 unsaturated)
acyl reduction
addition
polymerization
botryalyl ether (11)
condensation
Figure 3. Mechanism for biosynthesis of PRB-A and PRB-B by B. braunii. The numbered structures correspond to identically numbered compounds in Table 3.
Cell wall-associated hydrocarbon derivatives produced by various races of B. braunii
| Name | Producing race | Structure | |
| 1 | Botryals C52+xH98+2xO x = 0-12 even number |
Race- A/B | |
| 2. | Epoxy botryals | Race- A/B | |
| 3 | Alkenyl resorcinol | Race- A | |
| 4 | Alkenyl pyrogallol | Race- A | |
| 5 | Alkenyl phenol CxH2x-8O3 |
Race- A | |
| 6 | Alkenyl epoxides CxH2x-2O |
Race- A/B |
| 7 | Botryococcoid ether | Race- A | |
| 8 | Di-O-alkenyl ether | Race- A | |
| 9 | Alkenyl-O-alkyl phenol ethers | Race- A |
| 10 | Alkenyl-O-botryalyl ether | Race- A/B | |
| 11 | Alkenyl-O-epoxy botryalyl ether. | Race- A/B | |
| 12 | Tri acyl glycerol | Race- A | |
| 13. | Fatty acid | Race- A | |
| 14 | Trimethyl aldehyde | Race- A | |
| 15 | Trimethyl fatty acid | Race- A |
Botryals (1, as explained in Table 3) are even-numbered C52 to C64 α-branched, α-unsaturated aldehydes produced by aldol condensation of even C26 to C32 monounsaturated aldehydes. Botryals are analogous to mycolic acid (α-branched, β-OH acids) found in Corynebacterium (C32-C36), Nocardia (C34-C36) and Mycobacterium (C78-C85) species (Metzger and Casadevall, 1989). The trans isomer (-CHO group and bulkier groups are trans) predominates in botryals, which is consistent with acid/ base catalyzed aldol condensation mechanism. Alkenyls phenols (5), alkenyl resorcinols (3), and alkenyl pyrogallols (4) are synthesized via a common pathway, involving a tetraketide intermediate (Figure 4). This symmetrical intermediate undergoes decarboxylation to resorcinol derivatives that, depending on position of the oxidation, produce alkenyl pyrogallols and alkenyl phenols (Metzger and Pouet, 1995). These phenolic moieties protect the aliphatic chains in the A race against degradation by bacteria and fungi. Epoxidases, which have broad substrate specificities, are responsible for the formation of epoxides (6).
Botryococcoid ethers (7) constitute a substantial fraction of the biomass. Here a C27 alkadienyl secondary alcohol is linked to the -OH bearing carbon via another bridge to a C27 alkatrienyl chain (7a) or to a saturated n-C24/C26 alkyl chains (7b) (Metzger and Casadevall, 1991, 1992). A biosynthetic mechanism for the formation of botryococcoid ether by B. braunii has been proposed (Figure 5). Botryococcoid ethers and other ethers on repeated condensation produce a network of biopolymers. This mechanism includes ring opening of a protonated epoxide with specific cleavage of C-9'-O bond (Metzger and Casadevall, 1992). A resistant biopolymer (PRB-L) occurs in the L race also. This accounts for a larger fraction of total biomass than PRB-A and PRB-B do in A and B races. PRB-L structure is composed of saturated long (up to C30) hydrocarbon chains probably linked via ether bridges, and it is closely related to lycopadiene, the sole hydrocarbon produced by L race (Dernne et al., 1989). The presence of epoxylycopane (19), lycopanerol-A (20) (Metzger and Aumelas, 1997), lycopanerol B-G (Rager and Metzger, 2000), and aliphatic polyaldehyde tetraterpene diol polyacetal (Bertheas et al., 1999) in PRB-L is also reported.
V. Synthesis of other biochemicals
A. Exopolysaccharides
Microbial exopolysaccharides (EPS) are attracting much attention because of their numerous applications. Also, in some cases, the production of exopolysaccharides is linked with pathogenicity of microorganisms. The commercial usefulness of most industrial polysaccharides is based on their ability to alter rheological characteristics of solutions. Polysaccharides are used extensively in textiles, laundry products, adhesives, paper, paint, and foods. Many algae can produce exopolysaccharides, but few microalgal polysaccharides are used commercially. EPS produced by the alga Chlamydomonas mexicana can be used as a soil conditioner (Koren and Rayburn, 1984) and EPS synthesized by Porphyridium curentum is considered to be commercially important (Anderson and Eakin, 1985).
B. braunii is capable of synthesizing exopolysaccharides, as was first reported for the A race (Casadevall et al., 1985). Cells of B. braunii possess an internal fibrillar layer made of mucilaginous polysaccharides. This polysaccharide slowly dissolves in the culture medium, causing an increase in viscosity. The yield of EPS ranges from 250 g·m-3 for A and B races to 1 kg·m-3 for the L race. Galactose is the major component of the heterogeneous polysaccharide. Other detectable components of the polymer are fucose, arabinose, rhamnose, uronic acids, and unusual sugars such as 3-O-methyl fucose, 3-O-methyl rhamnose, and 6-O-methyl hexose. In contrast to A and B races, glucose is detected in significant amounts in the L race polysaccharides (Allard and Casadevall, 1990). B. braunii UC58, a strain isolated from a lake in Portugal, produces large amounts of EPS (4.0 to 5.5 kg·m-3) and low amounts of hydrocarbons (5%) (Fernandes et al., 1989). The polysaccharide production occurs both during growth and stationary phases, but higher production rates are seen as the growth rate declines. This enhanced production is associated with the development of nitrogen limitation in the culture medium. Under nitrogen starvation, the content of photosynthetic pigments in the cell decrease, and the rate of photosynthesis is reduced. Microalgae appear to respond to this condition by degrading nitrogen containing macromolecules and accumulating carbon reserve compounds, such as polysaccharides and fats.
Higher growth and production of EPS occur when nitrate is the nitrogen source instead of urea or ammonium salts. The consumption of urea and ammonium causes a rapid decline in pH, and this explains the relative poor growth and polymer production when using these nitrogen sources. The broth viscosity begins to decline when urea and ammonium salts are the nitrogen sources and the culture enters the stationary phase. This suggests acid and/or enzymatic hydrolysis of the biopolymer. During the stationary phase, polysaccharide hydrolyzing enzymes can be released by cell lysis. Nitrate at a concentration of 8 mM is apparently the optimal nitrogen source for obtaining extended growth (Lupi et al., 1994). Cells grown under continuous illumination exhibit higher EPS productivity (both volumetric and specific) than cells grown under cyclic illumination (Lupi et al., 1994). Optimum temperature range for EPS synthesis is 25 to 30°C, which is coincident with optimum temperature range for growth. Below 25°C EPS synthesis declines, although good growth can still be maintained.
The degree of polymerization of the biopolymer is significantly reduced when the temperature is outside the optimal range. During batch culture, the rheological behavior of the medium changes from Newtonian to non-newtonian because of EPS production during growth; however, in long-term culture the rheology reverts to Newtonian because of hydrolysis of the polymer (Lupi et al., 1991).
The associated bacteria (Auriobacterium barkeri, present in the non-axenic strain of B. braunii UC58 isolated from Portugal) have no effect on the production or stimulation of EPS synthesis (Fernandes et al., 1989). Supposedly, the affinity of certain bacterial species for sulfated glyco-conjugates exposed on epithelial cells of susceptible hosts can be reduced by microalgal exopolysaccharides. Sulfated polysaccharides of microalgae can be used in anti-adhesive therapies against bacterial infections both in cold-and warm-blooded animals. The adhesion of human pathogen Helicobacter pylori to the HeLaS3 human cell line is inhibited by various algal polysaccharides (Guzman-Murillo and Ascencio, 2000). Because it contains uronic acid, the EPS produced can be used to chelate metal ions and reduce metal-associated toxicity. Also, EPS is a potential source of fucose, a 6-deoxy sugar, which is a high-value substrate in chemical synthesis of flavoring agents (Lupi et al., 1991).
B. Alkanes
Methylated aldehydes are found in the cells of B. braunii (Metzger et al., 1991). These aldehydes are generated from fatty acids via methylation by S-adenosyl methionine and then converted to aldehydes by fatty acyl reductase. This enzyme requires ATP, CoA, and NADH for its activity. Fatty acyl reductase has been solubilized from microsomes by 0.1% octyl β-glucoside and purified to homogeneity by Blue A agarose and palmitoyl CoA agarose affinity column chromatography. The molecular weight of the enzyme was determined to be 35 kDa by SDS-PAGE. The N-terminal part of the enzyme is highly homologous with the fatty acyl reductase isolated from bacteria (Wang and Kolattukudy, 1995).
The aldehydes generated from fatty acids via methylation are converted to saturated hydrocarbons (alkanes). The resulting alkane has one less carbon than the aldehydes. The aldehyde to alkane conversion via decarboxylation requires decarbonylase activity under anoxic conditions. The decarbonylase enzyme is located in the microsome, and it is inactivated by 8-hydroxyquinoline, O-phenanthroline, and metal chelators such as EDTA. Inactivation by metal chelators reflects the involvement of metal ions with the activity of the enzyme. Plausibly, the metal ion is a part of the porphyrin complex of the decarbonylase enzyme. The inactivity of the enzyme in the presence of oxygen is reflected in an absence of alkanes when B. braunii is cultivated in aerobic conditions.
The decarbonylase of B. braunii also can convert carbon monoxide to carbon dioxide. This conversion of carbon monoxide, not reported in previously examined hydrocarbon synthesizing systems, may be necessary for organisms producing large amounts of hydrocarbons. The decarbonylase has a Michaelis-Menten constant (Km) value of 65 µM, a vmax of 1.36 nmol·min-1mg-1 and an optimum pH of 7.0 (Dennis and Kolattukudy, 1991).
C. Growth-promoting substances
Extracts of B. braunii biomass grown under certain conditions have been shown to have a growth-promoting effect on roots (Murakami, 2000). Growth promotion is achieved at extract concentrations as low as 50 ppm (Murakami, 2000).
VI. Role of bacterial association in hydrocarbon production
Interactions between bacteria and algae are well documented. Bacteria can stimulate algal growth by releasing substances such as vitamins and organic chelating agents (Haines and Guillard, 1974). Bacteria can produce assimilable nitrogen derivatives and inorganic nutrients for the algal cells (Parker and Bold, 1961). Also, bacteria can influence the pH and the redox potential by producing carbon dioxide (Parker and Bold, 1961). Bacteria can also inhibit algal growth by competing for limiting nutrients, as the nutrient uptake rates of algae are generally lower than those of bacteria (Saks and Kahn, 1979). Bacteria can secrete algaecides (Reim et al., 1974), degrade algal polysaccharides, and decompose algal cells (Fallon and Brock, 1979).
The presence of some accompanying microorganisms in B. braunii cultures was once claimed to be at least partly responsible for the observed production of hydrocarbons (Murry and Thomson, 1977). Studies of batch cultures of B. braunii kutzing (non-axenic strain) reveal that both the total biomass and hydrocarbon production at the onset of stationary phase are substantially lower when compared with axenic controls (Chirac et al., 1985). A lower hydrocarbon productivity in the presence of bacteria is linked with the hydrocarbon-degrading ability of the bacterial species associated with algal culture (especially Pseudomonas and Flavobacterium sp.) (Chirac et al., 1985). The various microbial flora associated with B. braunii are listed in Table 4.
Various microbial flora associated with B. braunii
| Strain | Source | Bacterial species associated |
| B. braunii (Cb) | Cambridge Culture Collection, UK | Arthrobacter sp. Corynebacterium sp. Pseudomonas sp. Erwinia sp. Alcaligenes sp. Flavobacterium sp. |
| B. braunii (Gb) | Gottingen Culture Collection, Germany | Streptomyces sp. |
| B. braunii (Tb) | Thonon Culture Collection, France | Streptomyces sp. |
Specific association effects have been studied by co-culturing axenic B. braunii (strain-A) with specific microorganisms such as Flavobacterium aquatile, Corynebacterium aquatile, Azotobacter chroococcum, and Pseudomonas oleovorans (Jones, 1972). These microorganisms were selected for various reasons: F. aquatile and C. aquatile occur commonly in aquatic environments and are known to associate with B. braunii, A. chroococcum can fix nitrogen, and P. oleovorans degrades hydrocarbons. When mixed cultures were aerated with CO2-enriched air, only F. aquatile caused enhancements in both the algal biomass and hydrocarbon production relative to axenic controls. P. oleovorans caused a marked reduction in algal biomass and hydrocarbon production.
Under CO2 limitation, the CO2 produced by bacterial respiration was used by the alga for photosynthesis. However, the amount of CO2 generated by bacteria was considerably lower than the algal demand. The beneficial effects of CO2 production outweighed the detrimental effects, and all the CO2-limited mixed cultures showed considerable increase in biomass and hydrocarbon production (Chirac et al., 1985).
Several bacteria exert considerable influence on growth yield and hydrocarbon production by B. braunii. The precise effects depend on culture conditions and the microbial species involved. The presence of Bacillus species and Corynebacterium species can stimulate hydrocarbon biosynthesis. In one case, the presence of Corynebacterium sp. raised the level of hydrocarbon of the algal biomass from 5.6% to 24.2% (Wang and Xie, 1996). The presence of microorganisms not only influences the quantity of hydrocarbons but also their relative abundance. Some microorganisms may alter the relative rates of synthesis of major hydrocarbons by B. braunii. However, the association with microorganisms is not essential for the alga to produce large quantities of hydrocarbons.
Not all bacterial species can readily associate with B. braunii. The inability to form stable association (revealed by a low population of associated species) can have several causes, including unsuitable culture conditions, inability of organic matter released by B. braunii to act as a good nutrient for growth, and antagonistic effects of B. braunii (Swale, 1968). In many cases, stable association will provide a large increase in algal biomass and hydrocarbon production, a reduced contamination by other microorganisms, and an improved supply of CO2.
VII. Bioreactor culture of B. braunii
A. Culture media and conditions
Much work has been done on establishing the optimal nutritional requirements and culture conditions for producing B. braunii hydrocarbons. The production of hydrocarbons in B. braunii appears to be growth associated, irrespective of the specific culture conditions and the nutrients used. Apparently, the observed slow growth rate of the alga is not a consequence of a limited supply of nutrients but the result of the substantial commitment by the cells to producing energetically expensive hydrocarbons.
Like other microalgae, B. braunii culture requires water, light, CO2, and inorganic nutrients. Culture productivity is affected by factors such as pH, pCO2, irradiance, salinity, and temperature. A modified Chu-13 medium is often used to culture B. braunii. This medium, at four-fold normal strength, has the following composition (kg·m-3): KNO3 (0.2), K2HPO4 (0.04), MgSO4·7H2O (0.1), CaCl2·6H2O (0.08), ferric citrate (0.01), citric acid (0.1), boron (0.5 ppm), manganese (0.5 ppm), copper (0.02 ppm), cobalt (0.02 ppm), and molybdenum (0.02 ppm). The pH is adjusted to 7.5 before sterilization (Largeau et al., 1980).
B. braunii cells grown in a luminostat culture in modified Prat medium attained a biomass concentration of 3.9 kg·m-3 and had a relatively short generation time of 3 to 4 days. The specific growth rate (µ) during exponential phase was 0.235 d-1. As the culture aged, it accumulated carbohydrates and lipids but lost some nitrogen-containing substances (Volova et al., 1998).
Photosynthetic cultures of B. braunii require CO2. Cultures aerated with 0.3% CO2-enriched air have a much shorter mass doubling time (40 h) compared with 6 days for cultures supplied with ambient air. CO2 enrichment favors the formation of lower botryococcenes (C30-C32), whereas cultures sparged with ambient air accumulate higher botryococcenes (C33-C34) (Wolf et al., 1985a). Apparently, methylation steps leading from C30 to C31 and C32 are faster in CO2-enriched cultures than steps leading to C33, C34 and higher homologues. Although autotrophic, B. braunii utilizes exogenous carbon sources for improved growth and hydrocarbon production. Various carbon sources, including C1-C6 compounds and disaccharides (lactose, sucrose), have been screened in attempts to decrease the mass doubling time of the alga from ≥1 week to less than 2 to 3 days (Weetal, 1985).
Although a deficiency of nitrogen favors lipid accumulation (Ben-Amotz et al., 1985), nitrogen is required for growth. Studies with nitrogen supplied as NO3–, NO2–, and NH3 reveal that the primary factor regulating nitrogen metabolism in B. braunii is the nitrate uptake system. Nitrogen is generally supplied as nitrate salts. An initial NO3 concentration of ≥0.2 kg·m-3 favors hydrocarbon production (Casadevall et al., 1983). With 1 kg·m-3 KNO3, the hydrocarbon concentration after 30 days was 4.8 kg·m-3. About the same amount of hydrocarbon (4.5 kg·m-3) was obtained if the initial concentration of KNO3 was 3 kg·m-3. This is because at high concentrations nitrate interferes with hydrocarbon production (Brenckman et al., 1989).
When the cells are exposed to 5 mM NH4+ for 24 h, the nitrate reductase enzyme becomes inactive. The use of NH4+ as the nitrogen source causes the culture pH to decline to less than pH 4, and this is damaging to cells. This NH4+-related toxicity manifests itself in the late exponential phase of growth, and the damage it causes is irreversible (Lupi et al., 1994). Photosynthesis and hydrocarbon production are drastically diminished when B. braunii (B race) cells are exposed to NH3. Total CO2 fixation declines by about 60% and oxygen evolution reduces by more than 50%. However, there is a pronounced increase in the synthesis of alanine, glutamine, and other amino acids, especially 5-aminolevulinic acid, a precursor of chlorophyll. This indicates a diversion of acetyl Co-A from hydrocarbon synthesis pathway to amino acid synthesis pathway (Ohmori et al., 1984).
Phosphorus is required for the growth of B. braunii, and it is usually supplied in the form of K2HPO4. Active growth persists after complete exhaustion of phosphate in the medium. In fact, phosphate levels can decline to below detection (0.5 g·m-3) early in the exponential phase (Casadevall et al., 1985). Many algae can rapidly absorb phosphate in amounts exceeding the requirement of the cell. This excess phosphate is stored as intracellular granules. The cells utilize on this phosphate reserve when the extracellular phosphate supply has run out.
In the final stages of culture, a large amount of phosphate can be released in the medium as the cells lyse. An increase in the initial amount of phosphate in the medium, beyond the level already present in the concentrated Chu-13 medium, does not affect growth, the nature of hydrocarbons, and their relative abundance. However, a noticeable increase in the amount of hydrocarbon produced has been reported in the presence of excess phosphate. This arises because of changes in the N:P ratio in the medium, and N:P ratio is known to influence the lipid content of various algae (Casadevall et al., 1985).
Studies have attempted to identify the optimal irradiance levels for supporting growth and hydrocarbon production. A high intensity of light increases the carotenoid-to-chlorophyll ratio, and this affects the color of algal colonies (Wolf et al., 1985a). Carbohydrate concentration, cellular nitrogen, and phosphorus content of B. braunii UTEX 572 are decreased by extended exposure to a light intensity of 25 to 72 µE·m-2s-1 (Oh et al., 1997).
Under intense illumination (10 klx, or ~140 µE·m-2s-1) algal cells that had been adapted to a high irradiance during preculture could attain a higher biomass concentration (7 kg·m-3) and hydrocarbon content (50% of dry weight) compared with cells that had been adapted to lowlevel irradiance (3 klx, or ~42 µE·m-2s-1) (Kojima and Zhang, 1999). The lower productivity of dark-adapted culture was because it was highly susceptible to photoinhibition. Photoinhibition could be reduced by partial shading of the bubble column photobioreactor (Kojima and Zhang, 1999). Biomass production could be doubled under continuous illumination (24 h) when compared with cultures that experienced a diurnal light cycle (12 h light/12 h dark); however, compared with diurnal illumination cycle, continuous light resulted in a fourfold increase in hydrocarbon production (Lupi et al., 1994).
Colony size increased with increased light intensity initially when the cell concentration was low and sufficient light for photosynthesis was available. As the cell concentration increased, the average light intensity within the photobioreactor decreased because of mutual shading (Mirón et al., 1999; Molina et al., 1999, 2000), leading to decrease in colony size. The production rates of extracellular polysaccharides and hydrocarbons decreased with decreasing average light intensity. It has been suggested that the equilibrium colony size is determined by a dynamic balance between the mechanical strength of colonies and the hydrodynamic stress due to turbulence in the reactors (Zhang and Kojima, 1998; Chisti, 1999).
The pH of the culture medium is generally adjusted to between pH 7.4 and 7.6 before inoculation. A regular increase in pH is observed during active growth followed by a slight decline later (Casadevall et al., 1985). The increase in pH is partly due to the consumption of dissolved CO2 for photosynthesis. Similar changes in pH are commonly observed in CO2-enriched cultures during exponential growth. The optimum temperature for growth is 25°C (Lupi et al., 1991). Sodium fluoride is found to stimulate growth when added at a concentration of 0.1 g·m-3 (Wang and Xie, 1996).
The biomass yield and the cellular composition of B. braunii race A are affected by the salinity (NaCl concentration) of the culture medium. A slight increase in the lipid content was observed as a result of increasing salinity until the salinity equaled that of seawater. Lower than normal content of protein, carbohydrates, and pigments were found in cells grown at 0.5 M NaCl (Ben-Amotz et al., 1985). Although the total lipid content of cells remained constant, the composition of lipids changed with NaCl concentration. In the lipid fraction the amount of hydrocarbons, acylglycerol, and phosphorus remained constant, but the hexose content (especially galactose) decreased. This was attributed to the consumption of UDP-galactose in the biosynthesis of osmoregulatory compounds. There was also a change in the fatty acid distribution in polar lipids, and the proportion of polyunsaturated fatty acids was higher than the saturated fatty acids when the salinity increased. The most important effect of saline stress was a proportional increase of α-laminaribose (o-β-D-glucopyranosyl-[1-3]-α-D-glucopyranose) content, which act as an osmoprotectant. The concentration of α-laminaribose tends to be lower in the Gottingen strain than in the Austin strain of the alga, and this explains the higher salt tolerance of Austin strain. Although organic osmoregulatory compounds (e.g., glycerol, glycine) are known to occur in algae, the osmoprotectory activity of α-laminaribose was first reported in B. braunii (Vanquez and Bertha, 1991).
Potentially, domestic sewage that has been pretreated by activated sludge treatment can be used as a medium for hydrocarbon production by B. braunii. Using sewage can reduce the cost of producing the hydrocarbons. Secondary stage-treated sewage (STS) has been characterized, and found to contain a large amount of nitrogen (as nitrate) and phosphorus (as PO43–). When B. braunii was cultured on STS aerated with 1% CO2 at 25°C and without controlling the pH, the nitrate content could be reduced from 7.67 g·m-3 to a level below detection (<0.01 g·m-3). The alga was able to consume PO43– present at quite low levels (0.02 g·m-3). NO2– appeared to be consumed after NO3–, but NH4+ was not utilized. The growth rate was 0.35 kg·m-3 per week and the hydrocarbon content was 53% compared with 58% in modified Chu-13 medium under the same conditions (Sawayama et al., 1992).
The use of B. braunii for tertiary treatment of municipal sewage and the conversion of the biomass to energy can reduce global warming and eutrophication. Botryococcus cannot grow on industrial wastewater containing a high concentration of inorganic ions. Attempts have been made to develop continuous biomass production processes using secondary-stage treated wastewater as the culture medium. A 2-l continuous flow bioreactor operated at a dilution rate of 0.57 d-1 attained a biomass productivity of 196 g·m-3 per week. The bioreactor was aerated with 1% CO2-enriched air (500 ml/min) and illuminated continuously at 68 µE·m-2s-1. Under these conditions, nitrates and phosphates decreased from 5.5 g·m-3 and 0.08 g·m-3 to 4.0 g·m-3 and 0.03 g·m-3, respectively (Sawayama et al., 1994). Potentially, the productivity of continuous algal culture can be improved by optimizing the dilution rate. The optimal dilution rate is expected to depend on the strength of wastewater and the intensity of illumination. Productivity can be enhanced further by culturing the cells at an optimal lightdark cycling frequency (Molina et al., 1999, 2000, 2001).
B. braunii grew well and was the dominant species in mixed cultures of other bacteria and algae (specially Oscillatoria) when cultivated on swine wastewater in an outdoor photobioreactor. At 25°C, the removal rates of chemical oxygen demand (COD), the total dissolved carbon (TDC), total nitrogen and phosphorus were 0.83, 0.61, 0.69, and 0.16 g·m-3 per day, respectively. The lipid content of the alga grown in swine wastewater and the modified Chu-13 medium were 32.8 ± 3.2% and 33.2 ± 2.6%, respectively (Lee et al., 1999).
B. Photobioreactors used for cultivation
Many kinds of photobioreactors are available for culturing microalgae (Molina et al., 1999; Mirón et al., 1999, 2000), and most of these have been evaluated for B. braunii culture. Batch and continuous schemes of operation have been tested in open and closed (axenic) bioreactors. Because of economics, only continuous culture is realistically feasible for the large-scale production of microalgal biomass. A commercially viable continuous culture process needs to attain a high density of biomass, or the biomass productivity will be unacceptably low. Because light penetration in dense cultures of microalgae is extremely limited, only thin channel flat plate photobioreactors and narrow bore tubular bioreactors are likely to be satisfactory for commercial production. The channel depth and tube diameter in such reactors would be generally ≤10 cm. Although thin channel flat plate types of photobioreactors are known to be highly efficient, they are not easily scaled up to sizes that will be necessary for producing significant quantities of hydrocarbons from B. braunii. This narrows the choice to tubular photobioreactors. Generally, tubular photobioreactors have provided high biomass productivities in closed cultures of microalgae (Molina et al., 1999; Acién Fernández et al., 2001). Tubular bioreactors require a large land area for a given volume of reactor, and this is a significant disadvantage.
Bubble columns and airlift reactors that are more compact than tubular devices can offer important advantages for large scale culture if a certain loss of productivity is accepted (Mirón et al., 1999, 2000). Continuous culture in airlift bioreactors (Chisti, 1989; Acién Fernández et al., 2001; Mirón et al., 1999, 2000) has been used especially frequently for producing B. braunii at a small scale (Casadevall et al., 1985; Bailliez et al., 1988). Maximum fixation of CO2 and hydrocarbon production by the alga were reported in a bubble column photobioreactor. The vessel (7 cm diameter) was constructed of glass, and it had a conical bottom. The culture was aerated with 1% CO2-enriched sterile air at a rate of 0.5 vvm. Illumination was provided using halogen lamps installed on one side of the reactor. The light intensity was 10 klx (approximately 140 µE·m-2s-1). The reactor could be partly shaded by an aluminum foil wrapped on the upper part. This achieved a certain light-dark cycling frequency (Molina et al., 1999, 2000, 2001; Mirón et al., 1999), to attempt to relieve photoinhibition (Casadevall et al., 1985). A biomass concentration of 7 kg·m-3 was attained, and the hydrocarbons constituted 50% of dry cell mass. Bubble columns have been claimed to subject the cells to a lesser hydrodynamic stress than similarly configured airlift photobioreactors (Kojima and Zhang, 1999), but this is debatable.
Compared with closed aseptic culture vessels, open pond reactors can provide a moderate surface-to-volume ratio at a much lower cost per unit volume (Weissman et al., 1988). However, the culture conditions in opens systems are less well controlled than in closed photobioreactors and, consequently, the biomass productivity is low compared with closed reactors. Also, open culture systems inevitably contain mixed populations and not just the desired alga. Notwithstanding these problems, open culture systems such as the “raceway” ponds may be the most realistic for producing large amounts of B. braunii for extracting hydrocarbons. This would be so especially if an inexpensive source of CO2 could be found to circumvent the relatively low CO2 utilization efficiency of open channels and ponds.
VIII. Use of Immobilized Algal Cells
Use of immobilized cell culture offers important advantages over free suspension culture especially when the cells are slow growing. Extracellular products can be recovered continuously from immobilized cell systems. Immobilization allows reuse of the biomass and eases separation of biomass from the suspending fluid. Also, immobilization can protect cells against hydrodynamic shear forces, and this can be important because microalgae can be shear-sensitive (Chisti, 1989, 1999). In view of these factors, many immobilization schemes have been examined for use with B. braunii cultures.
Immobilization in calcium alginate beads results in cells with enhanced chlorophyll content and photosynthetic activity relative to free controls. This behavior is seen at all stages of a batch culture under continuous illumination. Immobilization reduces the irradiance seen by the cells, and they respond by enhancing the chlorophyll content in attempts to capture more of the available light. Also, the gel-entrapped cells are protected from photoinhibition, a condition in which intense irradiance actually causes a loss in photosynthetic performance (Molina et al., 1999; Mirón et al., 1999).
Alginate gel-immobilized cells have lower growth rates and lower biomass production relative to free controls. This is possibly because immobilization can reduce access of nutrients to the cells and the immobilization matrix has a colony-confining effect. Immobilized cells retain the ability to produce hydrocarbons, whose structure and relative abundance are not affected by immobilization (Bailliez et al., 1986). In some cases, a notable increase in the hydrocarbon production (+20% compared with free cell system) has been recorded for immobilized cells, suggesting immobilization-related diversion of metabolic pathway toward hydrocarbon synthesis (Bailliez et al., 1985).
Immobilization by occlusion in polyurethane foams (PUF) has been used for B. braunii cells. PUF support matrices are versatile because of their broad range of porosities and mechanical properties. The immobilization of B. braunii cells in PUF via entrapment has led to reduced photosynthetic activity and hydrocarbon production in some cases. Nutrient and diffusion limitations are apparently not the cause of reduced performance (Bailliez et al., 1985), but light limitation is a plausible explanation. A study of PUF-immobilized Anabaena variabilis associated the observed poor performance with the toxic nature of the matrix. PUF matrix can release water-soluble toxic compounds (Gudin and Thomas, 1981). Also, increased local temperature and pressure in the matrix may explain some of the poor results. Some foam-matrix immobilized cultures can experience a substantial leakage (e.g., ~30%) of cells (Bailliez et al., 1988). One study observed that the hydrocarbon production of foamadsorbed cultures was comparable to that of freely suspended cells when cultivated in a airlift photobioreactor for 3 weeks (Bailliez et al., 1988). The average size of the foamadsorbed colonies remained quite small and allowed greater recovery of hydrocarbons from the culture by solvent extraction (Bailliez et al., 1988).
In one case, cotton gauze-immobilized B. braunii cells showed higher levels of hydrocarbon production, biomass growth, and photosynthetic activity when compared with cells immobilized in PUF (Yang and Wang, 1989). Despite advantages, the practicability of immobilized algal culture remains questionable unless the cells can be cultured in long-duration continuous culture and the hydrocarbons can be extracted continuously.
IX. Downstream processing
A. Hydrocarbon Recovery
Product recovery from fermentation and cell culture systems typically requires a series of unit operations that often control the economic viability of a production process (Chisti, 1998). The selection and design of a recovery scheme is related with the nature of the source organism and the properties of the product. Producing the biomass at a low cost can have a profound impact on the overall cost of product recovery (Belarbi et al., 2000), especially if the product does not need to be purified extensively.
Once the B. braunii biomass has been recovered from the broth by filtration or centrifugation, the hydrocarbons can be recovered either by pressing out or by the solvent extraction of the biomass. Pressing out the oils has been attempted on a laboratory scale, but no information is available on the extent of recovery. Because B. braunii has a thick cell wall and the wet biomass paste contains 10 times more water than hydrocarbons, recovery by pressing is not efficient. Also, this recovery method cannot be applied to continuous culture of immobilized cells.
Solvent extraction of biomass can remove the hydrocarbons without damaging the cells, if the solvent used is nontoxic. In addition, the selected solvent should have the following properties: the solvent should be immiscible with water; it should preferentially solubilize the product of interest; it should have a low boiling point for ease of removal; it should have a density that is significantly different than water; it should be readily available and inexpensive; and it should be reusable. In view of these considerations, hexane appears to be the solvent of choice for extracting algal hydrocarbons (Frenz et al., 1989a, b). For efficient extraction, the cells must first be concentrated to a paste because the presence of too much water around the cells reduces contact of the nonpolar solvent with the outer cell wall where the hydrocarbons are stored.
Under suitable conditions, 70% of hydrocarbons can be released by 30 min of contact with hexane. Growth and hydrocarbon production are not affected by repeated extraction with hexane. In fact, a higher content of hydrocarbons has been observed in hexane-treated biomass relative to controls. Recovery yields are influenced by physiological status of culture. In one case, cells in the early exponential growth phase permitted greater recovery of hydrocarbons when cultured in a airlift photobioreactor compared with culture in standard stirred bioreactor (Frenz et al., 1989a). This effect was explained by the smaller average size of colonies in the stirred vessel (Frenz et al., 1989a). The smaller average colony size in the stirred tank was probably caused by a higher turbulence in the tank than in the airlift reactor (Chisti, 1999).
Scale up of extraction can be difficult, because the alga tends to aggregate and form clumps during extraction. This happens because of differences in polarities of the solvent and the wet cells. Clumping shields a large fraction of the biomass from exposure to solvent. Agitation at high speed can overcome this problem, but intense agitation damages the cells (Chisti, 1999). Also, intense agitation can be quite expensive for large-scale commercial operations.
Hydrocarbons can be recovered continually from immobilized cells. Recovery yields are markedly increased relative to freely suspended controls when the cells are immobilized by adsorption in polyurethane foam (PUF). Because the colonies are at the surface of the immobilization matrix, they are easily accessed by the solvent. Optimal contact time was found to be 30 min when extracting with hexane (Frenz et al., 1989a, b).
Supercritical fluid (SCF) extraction is another technology that has been applied to B. braunii (Mendes et al., 1995). Because of relatively low values of viscosity, density, and surface tension, supercritical fluids such as CO2 allow rapid extraction of solids. Supercritical CO2 is nontoxic, inexpensive, easily removed from the extract, and reusable. A relatively low value of critical temperature allows use of CO2 for extracting thermolabile compounds. In the extraction of algal hydrocarbons with CO2 at 30°C, the solubility of hydrocarbons was observed to increase with increasing pressure (Mendes et al., 1995). The highest rate of extraction was obtained at a pressure of 30 MPa (Mendes et al., 1995).
B. Converting algal hydrocarbons and oil-rich biomass to fuels
Hydrocarbons obtained in the hexane extract of B. braunii can be burnt directly; however, for improved performance in internal combustion engines, the oil must be modified by processes such as pyrolysis and catalytic cracking.
Wet and dry algal biomass can be subjected to direct thermochemical liquefaction to produce combustible oil. Thermochemical liquefaction for 1 h (300°C, 10 MPa pressure) in the presence of 5% Na2CO3 as a catalyst provides a good yield of oil (Sawayama et al., 1999). This oil can be fractionated by silica gel column chromatography into three fractions, as follows: (1) a low-molecular weight (197 to 281 Da) fraction formed by the degradation of botryococcenes (C17-C22 hydrocarbons), constituting about 5% of total; (2) Botryococcenes with molecular weights of 438 to 572 Da, constituting 27.2% of the oil; (3) Polar substances (867 to 2209 Da molar mass) produced from organic material other than hydrocarbons and constituting 22.2% of the oil (Inoue, 1994). Thermochemical liquefaction of biomass eliminates the need for initial solvent extraction for recovery of hydrocarbons. However, the overall yield of oil from liquefied biomass is low (52.9%) compared with yield obtained by hexane extraction (Dote et al., 1992). Of course, the thermochemical processing of biomass is not applicable to continuous culture operations where all biomass is retained in the culture vessel and hydrocarbon extraction occurs continually in situ (Frenz et al., 1989a, b).
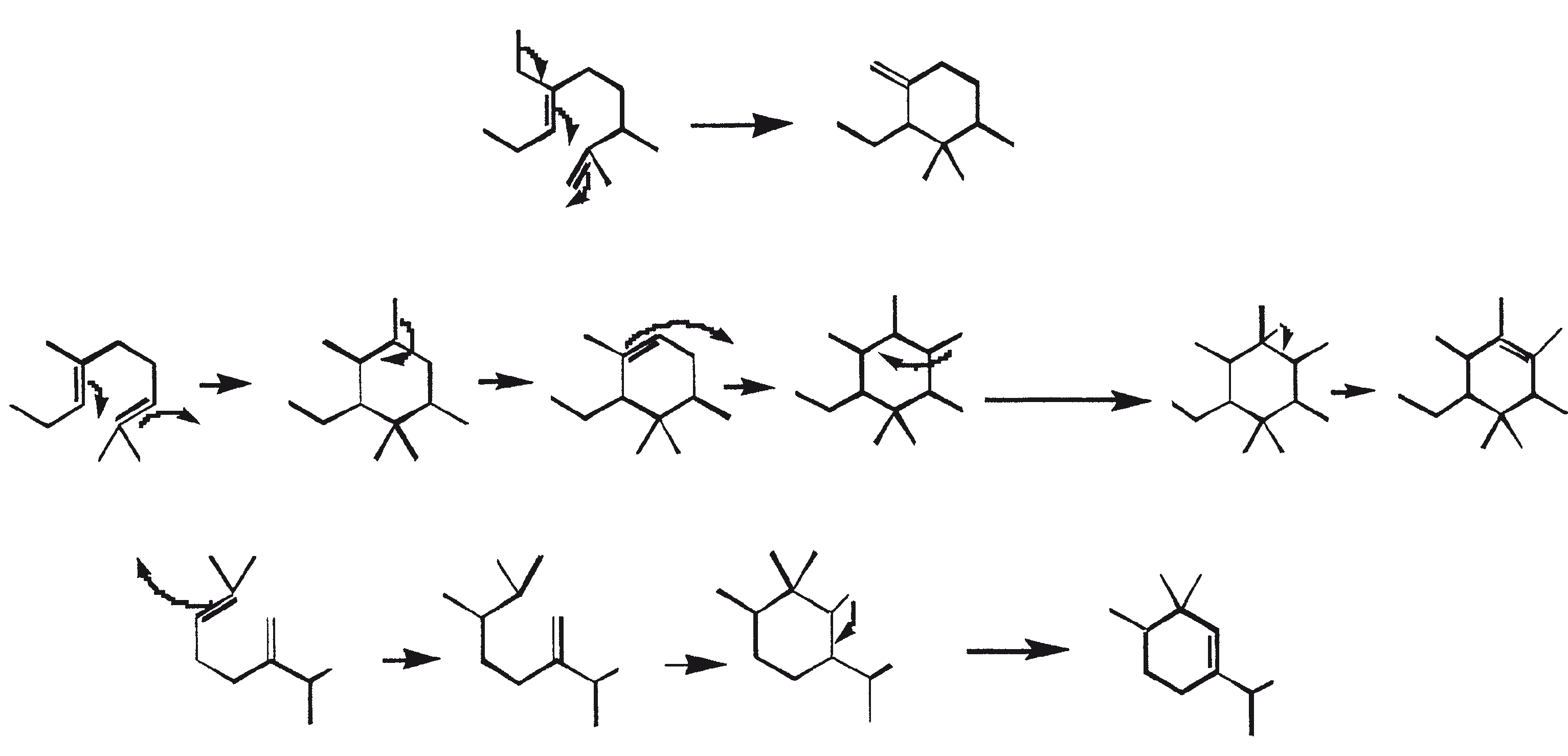
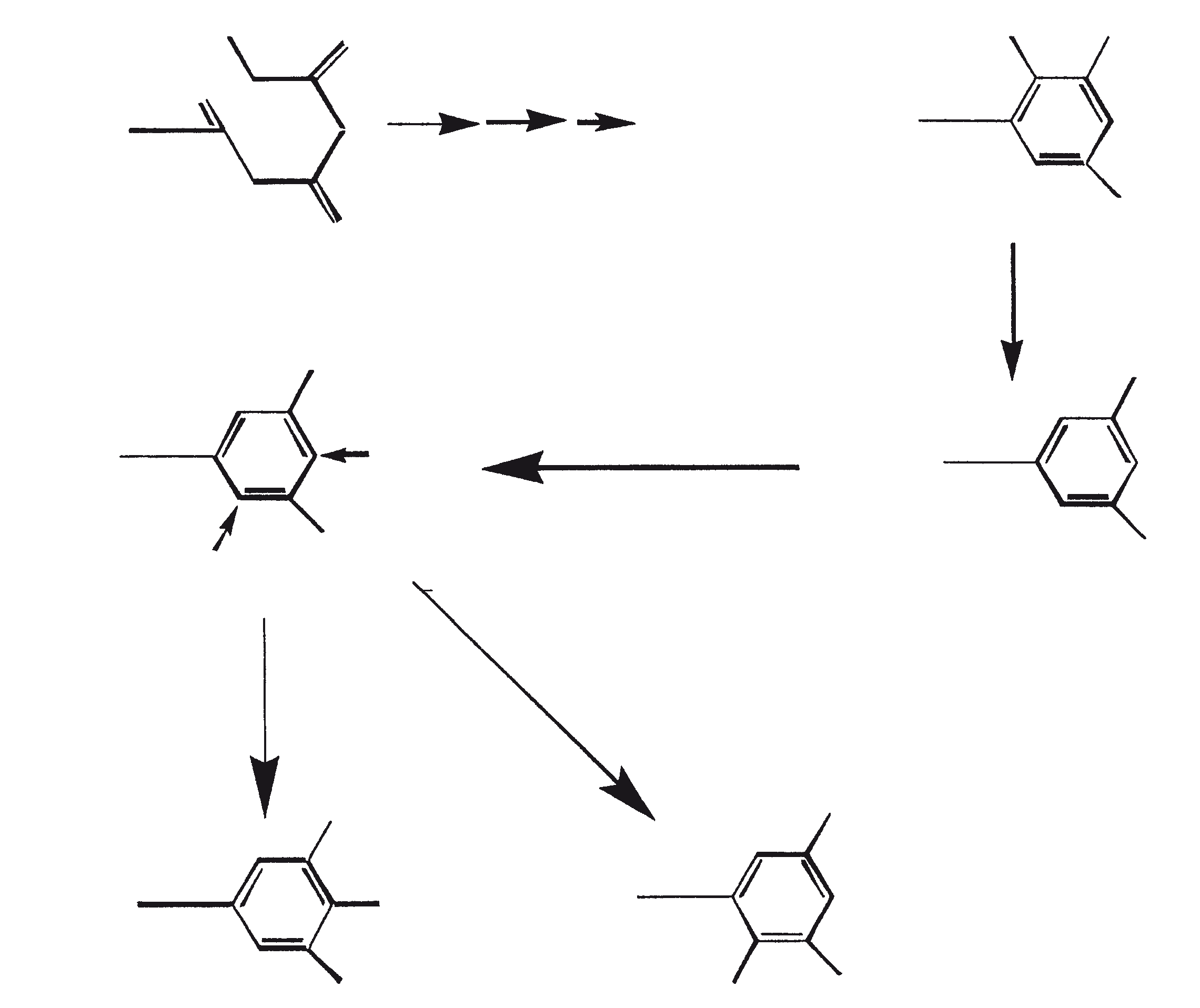
Crude hydrocarbons of B. braunii can be converted to gasoline (60 to 70%), light cycle oil (10 to 15%), heavy cycle oil (2 to 8%), and coke (5 to 10%) by subjecting the crude oil to catalytic cracking (Kitazato et al., 1989). A variety of cracking catalysts can be used, including CoMo catalyst (Held et al., 1985) and zeolites (Kitazato et al., 1989). Yield of gasoline and other fractions depend on the catalyst and the reaction temperature. The optimal conversion temperature with zeolite catalyst is 497°C. The yields of gasoline obtained by catalytic cracking of algal hydrocarbons are comparable to yields obtained from petroleum. Also, the gasoline produced has a sufficiently high octane number for direct use in automobiles. High octane numbers result because of the formation of alkylbenzenes, mainly xylenes and trimethyl-benzenes. These aromatic hydrocarbons are derived from botryococcenes (mainly the C34H58 compound) directly through consecutive cracking or cyclo-aromatization (Kitazato et al., 1989). Figure 6 provides a mechanistic representation of catalytic cracking of botryococcene, C34H58. A highly specific oxidation of the vinyl double bond in botryococcene (C34H58) has been used to convert this compound to botryococcone, C34H58O, a methyl ketone (Chisti, 1980).
X. Cloning of the hydrocarbon genes
Only a few reports exist on the cloning of B. braunii genes into other organisms. Recently a 366-bp cDNA fragment was obtained from the B race using reverse transcriptase/polymerase chain reaction (PCR) and degenerate primers based on conserved amino acid sequences found in all squalene synthase enzymes. Using that putative squalene synthase fragment as a probe, a 2632-bp cDNA clone was isolated from a cDNA library. The cDNA contained an open reading frame (ORF) coding for a protein with 461 amino acids and a molecular mass of 52.5 kDa. A comparison of Botryococcus squalene synthase (BSS) gene with that from other organisms showed a 52% homology with Nicotiana tabacum, 51% with Arabidopsis thaliana, 48% with Zea mays, 40% with rat, 39% with Saccharomyces cerevisiae, and 26% with Zymomonas mobilis (Okada et al., 2000).
The entire ORF region of BSS was amplified by PCR using a forward primer having NdeI restriction site and a start codon and a reverse primer having BclI site and stop codon to generate a 1403-bp fragment. A carboxy terminal truncated BSS of 1325 bp was also generated by PCR using the same forward primer and different reverse primer. The expression of full-length and carboxy terminal truncated BSS in E. coli BL21 in presence of isopropyl thio-β-D-galactoside (IPTG) resulted in significant levels of squalene synthase enzyme activity, but no botryococcene synthase activity.
Squalene synthase is an endoplasmic reticulum-associated enzyme with the carboxy terminus tethering the enzyme to the ER membrane (Robinson et al., 1993). The association with ER hampers the conventional purification of the enzyme. RNA blot hybridization analysis of algal cultures indicated preferential expression of BSS gene during rapid growth. Southern blot analysis indicated a single copy of SS gene in the algal genome (Okada et al., 2000). All these results suggest that either coordinated expression of separate synthase genes for squalene and botryococcenes exists or that there are physiological conditions controlling the squalene synthase vs. botryococcene synthase activity of a single polypeptide species.
XI. Concluding Remarks
In view of the material reviewed, B. braunii is a potentially good renewable source of useful hydrocarbons, polysaccharides, and other specialty chemicals. Catalytically cracked algal hydrocarbons have sufficiently high octane ratings for use as motor fuel. The use of algal hydrocarbons can greatly reduce the environmental impact associated with using coal and petroleum. Also, B. braunii can be used for removing low levels of contaminating nitrates and phosphates from wastewater.
At present, the production of photosynthetic fuel oils is not competitive with petroleumderived fuels. One reason for this is a relatively slow growth rate of B. braunii. The hydrocarbon synthesis genes of B. braunii have been cloned into other microorganisms. This can potentially increase the production rates and reduce problems associated with a high viscosity of B. braunii broths. Substantial developmental effort is needed to consistently culture B. braunii in large-scale systems.
References
Acién Fernández, F. G., Fernández Sevilla, J. M., Sánchez Pérez, J. A., Molina Grima, E., and Chisti, Y. 2001. Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: assessment of design and performance. Chem. Eng. Sci. 56: 2721.
Allard, B. and Casadevall, E. 1990. Carbohydrate composition and characterisation of sugars from the green microalga Botryococcus braunii. Phytochem. 29: 1875.
Anderson, D. B. and Eakin, D. E. 1985. A process for the production of polysaccharides from microalgae. Biotechnol. Bioeng. Symp. 15: 533.
Bailliez, C., Largeau, C., and Casadevall, E. 1985. Growth and hydrocarbon production of Botryococcus braunii immobilized in calcium alginate gel. Appl. Microbiol. Biotechnol. 23: 99.
Bailliez, C., Largeau, C., Berkaloff, C., and Casadevall, E. 1986. Immobilization of Botryococcus braunii in alginate, influence on chlorophyll content, photosynthetic activity and degeneration during batch culture. Appl. Microbiol. Biotechnol. 23: 361.
Bailliez, C., Largeau, C., Casadevall, E., Yang, L. W., and Berkaloff, C. 1988. Photosynthesis, growth and hydrocarbon production of Botryococcus braunii immobilized by entrapment and adsorption in polyurethane foams. Appl. Microbiol. Biotechnol. 29: 141.
Behrens, A., Schaeffer, P., Bernasconi, S., and Alrecht, P. 2000. 7,11-cyclobotryococca 5,12,26-triene, a novel botryococcene related hydrocarbon occurring in environment. Organic Lett. 2: 1271.
Belarbi, E. H., Molina, E., and Chisti, Y. 2000. A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzyme Microb. Technol. 26: 516.
Ben-Amotz, A., Torbene, T. G., and Thomas, W. H. 1985. Chemical profile of selected species of microalgae with emphasis on lipids. J. Phycol. 21: 72.
Bertheas, O., Metzger, P., and Largeau, C. 1999. A high molecular weight complex lipid, aliphatic polyaldehyde tetraterpene diol polyacetal from race L of Botryococcus braunii. Phytochemistry 50: 85.
Blackburn, K. B. 1936. Botryococcus and algal coal. Trans. R. Soc. Edin. 58: 841.
Brenckman, F., Largeau, C., Casadevall, E., and Berkaloff, C. 1989. Effect of nitrogen nutrition on growth and hydrocarbon production of the unicellular microalga Botryococcus braunii. Comm. Eur. Communities, Energy Biomass 717.
Brown, A. G. and Knights, B. A. 1969. Hydrocarbon content and it’s relationship to physiological state in the green alga Botryococcus braunii. Phytochemistry 8: 543.
Cane, R. F. 1977. Coorongite, balkashite and related substances—an annotated bibliography. Trans. R. Soc. S. Aust. 101: 153.
Casadevall, E., Largeau, C., Metzger, P., Chirac, C., Berkaloff, C., and Coute, A. 1983. Hydrocarbon production by unicellular microalga Botryococcus braunii. Biosciences 2: 129.
Casadevall, E., Dif, D., Largeau, C., Gudin, C., Chamount, D., and Desanti, O. 1985. Studies on batch and continuous culture of Botryococcus braunii: hydrocarbon production in relation to physiological state, cell ultrastructure and phosphate nutrition. Biotechnol. Bioeng. 27: 286.
Chirac, C., Casadevall, E., Largeau, C., and Metzger, P. 1985. Bacterial influence upon growth and hydrocarbon production of the alga Botryococcus braunii. J. Phycol. 21: 380.
Chisti, Y. 1980. An unusual hydrocarbon. J. Ramsay Society 27-28: 24-26.
Chisti, Y. 1989. Airlift Bioreactors, Elsevier, London.
Chisti, Y. 1998. Strategies in downstream processing. In Bioseparation and Bioprocessing: A Handbook, vol. 2, Subramanian, G., Ed., Wiley-VCH, New York, 3.
Chisti, Y. 1999. Shear sensitivity. In: Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis, and Bioseparation, vol. 5, Flickinger, M. C. and Drew, S. W., Eds., Wiley, New York, 2379.
David, M., Metzger, P., and Casadevall, E. 1988. Two cyclobotryococcenes from the B race of green alga Botryococcus braunii. Phytochemistry 27: 2863.
Dennis, M. W. and Kolattukudy, P. E. 1991. Alkane biosynthesis by decarbonylation of aldehyde catalyzed by a microsomal preparation from Botryococcus braunii. Arch. Biochem. Biophys. 287: 268.
Derenne, S., Largeau, C., Casadevall, E., and Connan, J. 1988. Mechanism of formation and chemical structure of coorongite. Structure and origin of the labile fraction. Fate of botryococcenes during early diagenesis. Org. Geochem. 13: 965.
Dernne, S., Largeau, C., Casadevall, E., and Berkaloff, C. 1989. Comparative study of rat and tobacco squalene. Phytochemistry 38: 1137.
Dote, Y., Sawayama, S., and Yokoyama, S. 1992. Liquefaction of hydrocarbon-rich microalga. Am. Chem. Soc. Div. Fuel Chem. 37: 1836.
Fallon, R. D. and Brock, T. D. 1979. Decomposition of blue green algal (cyanobacterial) blooms in lake Mendota, Wisconsin. Appl. Environ. Microbiol. 37: 820.
Fernandes, H. L., Tomme, M. M., Lupi, F. M., Fialho, A. M., Sa Correia, I., and Novais, J. M. 1989. Biosynthesis of high concentration of an exopolysaccharide during cultivation of the microalga Botryococcus braunii. Biotechnol. Lett. 11: 433.
Frenz, J., Largeau, C., and Casadevall, E. 1989a. Hydrocarbon recovery by extraction with a biocompatible solvent from free and immobilized cultures of Botryococcus braunii. Enzyme Microb. Technol. 11: 717.
Frenz, Z., Largeau, C., Csadevall, C., Kollerup, F., and Daugulis, A. J. 1989b. Hydrocarbon recovery and biocompatibility of solvents for extraction from cultures of Botryococcus braunii. Biotechnol. Bioeng. 34: 755.
Gelpi, E., Schneider, H., Mann, J., and Oro, J. 1970. Hydrocarbons of geochemical significance in microscopic algae. Phytochemistry 9: 603.
Goodway, G. W., Fawcett, P., Green, D., and Shaw, G. 1973. Sporopollenin formation in the ascospore wall of Neurospora crassa. Arch. Microbiol. 101: 145.
Grung, M., Metzger, P., and Liaaen-Jensen, S. 1989. Algal carotenoids, primary and secondary carotenoids in two races of green alga Botryococcus braunii. Biochem. Sys. Eco. 17: 263.
Grung, M., Metzger, P., and Liaaen-Jensen, S. 1994. Secondary carotenoids in Botryococcus braunii race L, new strain. Biochem. Sys. Eco. 22: 25.
Gu, P., Ishii, Y., Spencer, T. A., and Shechter, I. 1998. Function-structure studies and identification of three enzyme domain involved in the catalytic activity in rat hepatic squalene synthase. J. Biol. Chem. 273: 12515
Gudin, C. and Thomas, D. 1981. Production de polysaccharides sulfates par un biophotoreacteur a cellules immobilisees de Porphyridium curentum. C. R. Sci. Paris 293: 35.
Gudin, C., Bernard, A., Chaumont, D. T., Thepnier, C., and Hardy, T. 1984. Direct conversion of solar energy into organic chemicals. World Biotech. Rep. 1: 541.
Guzman-Murillo, M. A. and Ascencio, F. 2000. Anti adhesive activity of sulfated polysaccharides of microalgae on attachment of red sore diseaseassociated bacteria and Helicobacter pylori to tissue culture cells. Lett. Appl. Microbiol. 30: 473.
Haines, K. C. and Guillard, R. R. L. 1974. Growth of vitamin B12 requiring marine diatoms in mixed laboratory cultures with vitamin B12 producing marine bacteria. J. Phycol. 10: 245.
Held, W., Peters, M., Buhs, C., Oelert, H. H., Reifenstahl, G., and Wagner, F. 1985. Production of hydrocarbon from biomass. Comm. Eur. Communities, Energy Biomass 744.
Huang, Z., Poulter, C. D., Wolf, F. R., Somers, T. C., and White, J. D. 1988. Braunicene, a novel cyclic C32 isoprenoid from Botryococcus braunii. J. Am. Chem. Soc. 110: 3959.
Huang, J. and Poulter, C. D. 1989. Tetramethyl squalene, a triterpene from Botryococcus braunii var showa. Phytochem. 28: 1467.
Inone, H., Korenaga, T., Sagami, H., Koyama, T., Sugiyama, H., and Ogura, K. 1993. Formation of farnesal and 3-hydroxy-2,3-dihydrofarnesal from farnesol by protoplasts of Botryococcus braunii. Biochem. Biophys. Res. Commun. 196: 1401.
Inone, H., Korenaga, T., Sagami, H., and Ogura, K. 1994a. Phosphorylation of farnesol by a cell free system of Botryococcus braunii. Biochem. Biophys. Res. Commun. 200: 1036.
Inoue, S., Dote, Y., Sawayama, S., Minowa, T., Ogi, T., and Yokoyama, S. 1994b. Analysis of oil derived from liquefaction of Botryococcus braunii. Biomass Bioenergy 6: 269.
Jarstfer, M. B., Blagg, B. S. J., Rogers, D. H., and Poulter, C. D. 1996. Biosynthesis of squalene. Evidence for a tertiary cyclopropylcarbinyl cationic intermediate in the rearrangement of presqualene diphosphateto squalene. J. Am. Chem. Soc. 118: 13089.
Jones, J. G. 1972. Studies on freshwater bacteria: association with algae and alkaline phosphatase activity. J. Ecol. 60: 59.
Kitazato, H., Asaoka, S., and Iwamoto, H. 1989. Catalytic cracking of hydrocarbons from microalgae. Sekiyu Gakkaishi 32: 28.
Kojima, E. and Zhang, K. 1999. Growth and hydrocarbon production by microalga Botryococcus braunii in bubble column photobioreactor. J. Bioscience Bioeng. 87: 811.
Koren, K. M. and Rayburn, W. R. 1984. Influence of growth status and nutrients on exocellular polysaccharides synthesis by the soil alga Chlamydomonas mexicana. J. Phycol. 20: 253.
Lareillard, J., Largeau, C., and Casadevall, E. 1988. Oleic acid in the biosynthesis of resistant polymer of Botryococcus braunii. Phytochemistry 27: 2095.
Largeau, C., Casadevall, E., Berkaloff, C., and Dhamliencourt, P. 1980. Sites of accumulation and composition of hydrocarbons in Botryococcus braunii. Phytochemistry 19: 1043.
Lee, S. J., Kim, S. B., Kim, H. S., Kwon, G. S., Yoon, B. D., and Oh, H. M. 1999. Mass cultivation of Botryococcus braunii for advanced treatment of swine waste water and lipid production in photobioreactor. Sanop Misaengmul Hakhoechi 27: 166.
Lichitfouse, E., Derenne, S., Mariotti, A., and Largeau, C. 1994. Possible algal origin of long chain odd n-alkanes in immature sediments as revealed by distribution and carbon isotope ratios. Org. Geochem. 22: 1023.
Lupi, F. M., Fernandes, H. M. L., Sa Correia, I., and Novais, J. M. 1991. Temperature profiles of cellular growth exopolysaccharide synthesis by Botryococcus braunii. J. Phycol. 3: 35.
Lupi, F. M., Fernandes, H. M. L., Tomme, M. M., Sa Correia, I., and Novais, J. M. 1994. Influence of nitrogen source and photoperiod on exopolysaccharide synthesis by the microalga Botryococcus braunii. Enzyme Microb. Technol. 16: 546.
Mcirdy, D. M., Cox, R. E., Vlkman, J. K., and Howel, V. J. 1986. Botryococcane in a new class of Australian non marine crude oils. Nature 320: 57.
Mendes, R. L., Coelho, J. P., and Fernandes, H. L. 1995. Application of supercritical CO2 extraction to microalgae and plants. J. Chem. Technol. Biotechnol. 62: 53.
Metzger, P., Berkaloff, C., Casadevall, E., and Coute, A. 1985a. Alkadiene and botryococcene producing races of wild strains of Botryococcus braunii. Phytochem. 24: 2305.
Metzger, P., Casadevall, E., Pouet, M. J., and Pouet, Y. 1985b. Structure of some botryococcenes: branched hydrocarbons from the B race of the green alga Botryococcus braunii. Phytochem. 24: 2995.
Metzger, P. and Casadevall, E. 1987. Lycopadiene, a tetraterpenoid hydrocarbon from new strains of the green alga Botryococcus braunii. Tetrahedron Lett. 24: 2305.
Metzger, P., David, M., and Casadevall, E. 1987. Biosynthesis of triterpenoid hydrocarbons in the B race of green alga Botryococcus braunii. Sites of production and nature of methylating agent. Phytochem. 26: 129.
Metzger, P., Casadevall, E., and Coute, A. 1988. Botryococcene distribution in strains of green alga Botryococcus braunii. Phytochemistry 27: 1383.
Metzger, P. and Casadevall, E. 1989. Aldehydes, very long chain alkenyl phenols, epoxides and other lipids from an alkadiene producing strain of Botryococcus braunii. Phytochemistry 28: 2097.
Metzger, P., Allard, B., Casadevall, E., Berkaloff, C., and Coute, A. 1990. Structure and chemistry of a new chemical race of Botryococcus braunii (chlorophyceae) that produces lycopadiene, a tetraterpenoid hydrocarbon. J. Phycol. 26: 258.
Metzger, P. and Casadevall, E. 1991. Botryococcoid ethers, ether lipids from Botryococcus braunii. Phytochemistry 30: 1439.
Metzger, P., Villarreal-Rosales, E., and Casadevall, E. 1991. Methyl branched fatty aldehydes and fatty acids in Botryococcus braunii. Phytochemistry 30: 185.
Metzger, P. and Casadevall, E. 1992. Ether lipids from Botryococcus braunii and their biosynthesis. Phytochemistry 31: 2341.
Metzger, P. and Pouet, Y. 1995. n-Alkyl pyrogallol dimethyl ethers, aliphatic diol monoesters and some minor ether lipids from Botryococcus braunii (A race). Phytochemistry 40: 543.
Metzger, P. and Aumelas, A. 1997. Lycopanerol-A, di-tetraterpenoid tetraether derivatives from green microalga Botryococcus braunii L race. Tetrahedron Lett. 38: 2977.
Mirón, A. S., Gómez, A. C., Camacho, F. G., Grima, E. M., and Chisti, Y. 1999. Comparative evaluation of compact photobioreactors for large-scale monoculture of microalgae. J. Biotechnol. 70: 249.
Mirón, A. S., Camacho, F. G., Gómez, A. C., Grima, E. M., and Chisti, Y. 2000. Bubble column and airlift photobioreactors for algal culture. AIChE J. 46: 1872.
Moldwan, J. M. and Seifert, W. K. 1980. First discovery of botryococcane in petroleum. J. C. S. Chem. Commun. 1: 912.
Molina, E., Fernández, F. G. A., Camacho, F. G., and Chisti, Y. 1999. Photobioreactors: light regime, mass transfer, and scaleup. J. Biotechnol. 70: 231.
Molina, E., Acién Fernández, F. G., García Camacho, F., Camacho Rubio, F., and Chisti, Y. 2000. Scale-up of tubular photobioreactors. J. Appl. Phycol. 12: 355.
Molina, E., Fernández, J., Acién, F. G., and Chisti, Y., 2001. Tubular photobioreactor design for algal cultures. J. Biotechnol. 92: 113.
Murakami, M., Nakano, H., Yamaguchi, K., Konosu, S., Nakayama, O., Matsumoto, Y., and Iwamoto, H. 1988. Meijicoccene, a new cyclic hydrocarbon from Botryococcus braunii. Phytochemistry 27: 455.
Murakami, N. 2000. Plant growth regulator composition and their manufacture. Jpn. Kokai Tokkyo Koho 264: 809.
Murry, J. and Thomson, A. 1977. Hydrocarbon production in Anacystis montana and Botryococcus braunii. Phytochemistry 16: 465.
Oh, H. M., Kim, S., Park, E. R., Lee, S. T., Kwon, J. S., and Yoon, B. D. 1997. Effects of light intensity and nutrients on the growth of Botryococcus sp. Misaengmul Hakhoechi 25: 339.
Ohmori, M., Wolf, F. R., and Bassham, J. A. 1984. Botryococcus braunii carbon/nitrogen metabolism as affected by ammonia addition. Arch. Microbiol. 140: 101.
Okada, S., Mastuda, M., and Yamaguchi, K. 1996. Botryoxanthin-A a new member of the new class of carotenoids from green microalga Botryococcus braunii, Berkeley. Tetrahedron Lett. 37: 1065.
Okada, S., Tonegawa, I., Mastuda, H., Murakami, M., and Yamaguchi, K. 1997. Carotenoids from green microalga Botryococcus braunii. Tetrahedron 53: 11307.
Okada, S., Tonegawa, I., Mastuda, H., Murakami, M., and Yamaguchi, K. 1998. Botryoxanthin-B and alpha botryoxanthin-A from green microalga Botryococcus braunii. Phytochemistry 47: 1111.
Okada, S., Devarenne, T. P., and Chappel, J. 2000. Molecular characterization of squalene synthase from the green alga Botryococcus braunii, race B. Arch. Biochem. Biophys. 373: 307.
Parker, B. C. and Bold, H. C. 1961. Biotic relationships between soil algae and other microorganisms. Am. J. Botany 48: 185.
Poulter, C. D. and Hughes, J. M. 1977. Model studies of the biosynthesis of non-head to tail terpenes. Stereochemistry of ionization for N-methyl-4[(1S, 1’R, 3’R)-[1-2H] chrysanthemyloxy]pyridinium iodide. J. Am. Chem. Soc. 99: 3824.
Poulter, C. D., Marsh, L., Argyle, J. C., Dennis, M. S., Robyn, J. G., and Scott, G. M. 1977. Model studies of biosynthesis of non-head to tail terpenes. Rearrangements of the chrysanthemyl system. J. Am. Chem. Soc. 99: 3816.
Rager, M. N. and Metzger, P. 2000. Six novel tetraterpenoid ethers, lycopanerols B-G, and some other constituents from green microalga Botryococcus braunii. Phytochemistry 54: 427.
Reim, R. L., Shane, M. S., and Cannon, R. E. 1974. The characterization of a Bacillus capable of blue green bactericidal activity. Can. J. Microbiol. 20: 981.
Robinson, G. Y., Tsay, Y. H., Kienzle, B. K., Smith-Monroy, C. A., and Bishop, R. W. 1993. Conservation between human and fungal squalene synthases: similarities in structure, function and regulation. Mol. Cell Biol. 13: 2706.
Saks, N. M. and Kahn, E. G. 1979. Substrate competition between a salt marsh diatom and a bacterial population. J. Phycol. 15: 17.
Sawayama, S., Minowa, T., Dote, Y., and Yokoyama, S. 1992. Growth of the hydrocarbon rich microalga Botryococcus braunii in secondarily treated sewage. Appl. Microbiol. Biotechnol. 38: 135.
Sawayama, S., Inone, S., and Tokoyama, S. 1994. Continuous culture of hydrocarbon rich microalga Botryococcus braunii secondarily treated sewage. Appl. Microbiol. Biotechnol. 41: 729.
Sawayama, S., Inoue, S., and Yokoyama, S. 1995. Phylogenetic position of Botryococcus braunii based on small subunit of ribosomal RNA sequence data. J. Phycol. 31: 419.
Sawayama, S., Minowa, T., and Yokoyama, S. 1999. Possibility of renewable energy production and carbon dioxide mitigation by thermochemical liquefaction of microalgae. Biomass Bioenergy 17: 33.
Swale, E. M. F. 1968. The phytoplankton of Oakmere, Cheshire. Br. Phycol. Bull. 3: 441.
Templier, J., Largeau, C., and Casadevall, E. 1984. Hydrocarbon formation in the green alga Botryococcus braunii and mechanism of nonisoprenoid hydrocarbon biosynthesis. Phytochem. 23: 1017.
Templier, J., Largeau, C., and Casadevall, E. 1991a. Biosynthesis of n-alkatrienes in Botryococcus braunii. Phytochemistry 30: 2209.
Templier, J., Largeau, C., and Casadevall, E. 1991b. Non-specific elongation-decarboxylation in biosynthesis of cis and trans-alkadienes by Botryococcus braunii. Phytochemistry 30: 175.
Templier, J., Disendorf, C., Largeau, C., and Casadevall, E. 1992a. Metabolism of n-alkadienes in the A race of Botryococcus braunii. Phytochemistry 31: 113.
Templier, J., Largeau, C., and Casadevall, E. 1992b. Chemical inhibition of resistant biopolymers in the outer walls of A and B races of Botryococcus braunii. Phytochemistry 31: 4097.
Templier, J., Largeau, C., and Casadevall, E. 1993. Variation in external and internal lipid associated with inhibition of resistant biopolymer from the A race of Botryococcus braunii. Phytochemistry 33: 1079.
Tonegawa, I., Okada, S., Murakami, M., and Yamaguchi, K. 1998. Pigment composition of the green microalga Botryococcus braunii, Kawaguchi. Fish Sc. 64: 305.
Vanquez, R. and Bertha, O. 1991. Haloadaption of the green alga Botryococcus braunii. Phytochemistry 30: 2919.
Volova, T. G., Kalacheva, G. S., Zhilo, N. O., and Plotnicov, V. F. 1998. Physiological and biochemical properties of the alga Botryococcus braunii. Russ. J. Plant. Physol. 45: 725.
Wake, L. V. and Hillen, L. W. 1981. Nature and hydrocarbon content of the blooms of the alga Botryococcus braunii occurring in Australian fresh water lakes. Aust. J. Mar. Freshwater Res. 32: 353.
Wang, X. and Kolattukudy, P. E. 1995. Solubilization and purification of aldehyde generating fatty acyl-CoA reductase from green alga Botryococcus braunii. FEBS Lett. 370: 15.
Wang, X. and Xie, S. 1996. Effects of several factors on growth of Botryococcus braunii. Weishengwuxue Tongbao 23: 275.
Weetal, H. H. 1985. Studies on nutritional requirements of the oil producing alga Botryococcus braunii. Appl. Biochem. Biotechnol. 11: 377.
Weissman, J. C., Goebel, R. P., and Benemann, J. R. 1988. Photobioreactor design: mixing, carbon utilization, and oxygen accumulation. Biotechnol. Bioeng. 31: 336.
Wolf, F. R., Nanomura, A. M., and Bassham, J. A. 1985a. Growth and branched hydrocarbon production in a strain of Botryococcus braunii. J. Phycol. 21: 388.
Wolf, F. R., Nemethy, E. K., Blanding, J. H., and Bassham, J. A. 1985b. Biosynthesis of unusual acyclic isoprenoids in the green alga Botryococcus braunii. Phytochemistry 24: 733.
Yang, L. W. and Wang, X. Y. 1989. Studies on hydrocarbon production by immobilized Botryococcus braunii cells. Rev. Roum. Chim. 34: 397.
Yong, T. P. C., Largeau, C., and Casadevall, E. 1986. Biosynthesis of non-isoprenoid hydrocarbons by the microalga Botryococcus braunii. Evidences for elongation- decarboxylation mechanism. Nouv. J. Chim. 10: 701.
Zhang, D. and Poulter, C. D. 1995. Biosynthesis of non head to tail isoprenoid. Synthesis of 1'-1 and 1'-3 structures by recombinant yeast squalene synthase. J. Am. Chem. Soc. 117: 1641.
Zhang, K. and Kojima, E. 1998. Effect of light intensity on colony size of microalga Botryococcus braunii in bubble column photobioreactor. J. Fermentation Bioeng. 86: 573.